
Int J Curr Pharm Res, Vol 17, Issue 6, 20-24Original Article
DEMOGRAPHIC DISTRIBUTION AND ADVERSE DRUG REACTION PATTERN OF ANTI-TUBERCULAR AND ANTIDIABETIC THERAPY IN PATIENTS OF TUBERCULOSIS WITH DIABETES MELLITUS: A PROSPECTIVE OBSERVATIONALSTUDY AT TERTIARY CARE HOSPITAL
JEENAL MISTRY1, ANITA SINHA2, B. DIVAKAR3, MRUGANK PATEL4*, NAYAN GAVLI5
1Department of Pharmacology, GMERS Medical College, Navsari-396445, Gujarat, India. 2,3,4Department of Pharmacology, Government Medical College, Surat-395001, Gujarat, India. 5Department of General Medicine, Bardoli Hospital, Dhuliya Chokdi, Bardoli Mahuva Road Bardoli, Surat-394601, Gujarat, India
*Corresponding author: Mrugank Patel; *Email: mrugankalbatross@gmail.com
Received: 12 Aug 2025, Revised and Accepted: 02 Oct 2025
ABSTRACT
Objective: The objectives of present study to evaluate the prevalence of diabetes mellitus among tuberculosis patient at a tertiary health care center and to study the adverse drug reaction due to simultaneous use of anti-tubercular drugs with anti-diabetic drugs.
Methods: A prospective observational cohort study was conducted from March 10, 2021, to July 9, 2022, at a tertiary care hospital, involving a total of 134 patients. The patient was approached as per NTEP guidelines. Age, gender, occupation, weight, and socioeconomic position were among the demographics that were noted and thoroughly examined. The screening for diabetes mellitus was done in patients with newly diagnosed tuberculosis. The adverse drug reactions due to the simultaneous use of antitubercular drugs with antidiabetic drugs were observed and compared with patients with tuberculosis without diabetes mellitus according to outcome parameters.
Results: Glycosylated hemoglobin (HbA1c) was used to screen for diabetes mellitus in a representative sample of TB patients. Patients who self-reported having DM or whose HbA1c was ≥6.5% were considered diabetics. Out of the 323 newly diagnosed TB patients that were examined, 67 (18%) had diabetes mellitus; 31 (8%) had DM that they had previously identified, and 36 (10%) had just received a diagnosis. Men, whose average age was 47, had a higher prevalence (P=0.00001). 31 (23.13%) of the 134 TB patients who had previously been diagnosed with diabetes mellitus had an HbA1c of 10%, which indicates inadequate glycemic management. In the summarized result, we can observe that the prevalence rate of DM is 20.74% among TB patients. Compared to other research, this is higher. Over the course of the study, 35 ADRs were identified, recorded, evaluated, and reported. Assessment of the severity of the suspected ADRs revealed that 28.57% of suspected ADRs were severe and 71.42% of ADRs were moderate in severity as per the Modified Hartwig and Siegel Severity Assessment Scale. On the causality assessment by the WHO scale, most ADRs were classified as “possible” among both groups. Most patients who experienced adverse drug reactions were older than 50. The most frequently impacted system was the gastrointestinal tract (9.7%). As per the Modified Schumock and Thornton Preventability Assessment Scale, the preventability of ADRs was assessed, and the results revealed that 11.19% of ADRs were preventable.
Conclusion: This study emphasizes that all newly diagnosed tuberculosis patients should be screened for diabetes mellitus, as there is a high prevalence of diabetes mellitus in tuberculosis patients. The concomitant use of anti-tubercular therapy and anti-diabetic therapy can cause adverse drug reactions as each group interacts with the other. Also,anti-tubercular therapy and anti diabetic therapy alone can cause adverse drug reactions.
Keywords: Anti-tubercular therapy, Anti diabetic therapy, Tuberculosis, Diabetes mellitus, Glycemic control
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijcpr.2025v17i6.7060 Journal homepage: https://innovareacademics.in/journals/index.php/ijcpr
INTRODUCTION
In developing countries due to increased burden of Diabetes Mellitus (DM), the risk of developing Tuberculosis (TB) almost triples in comparison to developed countries [1].
In India under NTEP (National Tuberculosis Elimination Program), in 2019, among the notified TB patients, 64% had their blood sugar screened. Among all TB patients screened, 7% were TB-Diabetes comorbid and 52% among these were linked on diabetic treatment [2].
Anti-tubercular drugs interact with anti-diabetic drugs [1]. Therefore, in patient of TB it needs to be known which anti-diabetic drugs are safe and not interacting or causing less adverse drug reaction, for better outcomes in patients of Tuberculosis with DM [3].
An analysis of nutrition and DM changes in India also suggests that increase in prevalence of DM contributed to an increase in total number of TB cases in the country which exceeded the rate of population growth during the same time period [1].
This study helps to better the existing data regarding knowledge about demographic profile and the prevalence of diabetes in tuberculosis patients and adverse drug reactions (if any).
MATERIALS AND METHODS
Study design
This prospective study was conducted at tertiary care hospital as per NTEP (National tuberculosis elimination program) guidelines. All patients coming to OPD and IPD of respiratory medicine at tertiary health care center were screened for tuberculosis. After confirmation of tuberculosis and/or tuberculosis with diabetes mellitus of two groups, patients were approach for informed consent and to be part of the study. Demographic details like age, gender, occupation, weight, marital status and socio-economic status were recorded and studied in detail. All investigation, reports and treatment details were observed and noted.
Inclusion criteria
Male and female patients, age ≥18 y
Patients of tuberculosis with or without Diabetes Mellitus as comorbidity.
Patients on treatment of anti-tubercular drugs with or without anti-diabetic drugs.
Patients willing to be part of the study.
Exclusion criteria
Pregnant woman
Patient with any other chronic disease (Hypertension, congestive heart failure, infective endocarditis, myocardial infraction, epilepsy, cerebrovascular stroke, meningitis, HIV, HBsAG, HCV, HAV, kidney failure, hepatitis etc).
The screening of diabetes mellitus was done in patients of tuberculosis. The adverse drug reactions due to simultaneous use of anti-tubercular drugs with anti-diabetic drugs were observed and were compared with patients of tuberculosis without diabetes mellitus. The adverse drug reactions noted and reported via ADR reporting form 1.4. Sample size was calculated based on non-probability purposive or judgmental criteria.
Statistical analysis
Analyses were performed using SPSS (Statistical Package for Social Sciences) software v29, with a significance level of p<0.05. All normally distributed data are analyzed by mean+/-SD. If not normally distributed, then by median and percentile. Qualitative data are analyzed by Chi-square test and proportion. Quantitative data are analyzed by student’s t-test and percentage. Univariate and multivariate logistic regression is used for analyzed by odds ratio, relative risk and attributable relative risk.
RESULTS
During the study period, 134 patients enrolled out of 323 in total that satisfied the study's inclusion and exclusion criteria requirements. In our study, 67 patients with diabetes mellitus and tuberculosis received anti-tubercular therapy in addition to anti-diabetic medications (ATT+ADD), And 67 patients received anti-tubercular therapy (ATT) only.
Overall age-wise distribution
In the age-wise distribution of the study population, we were found that maximum number of the patients in the study belonged to the age group 31-50 y mean age of the patients on ATT with ADD was 47.49 y with SD 13.37 y and mean age of the patients on ATT only was 33.24 y with SD 13.78 y (table 1).
Statistical analysis with Chi-square test gives χ2: 36.1561 with p-Value: 0.00001 suggesting high statistical significance.
Table 1: Overall age distribution (n=134)
| Age | Patients on ATT+ADD | Patients on ATT | ||
| n | % | n | % | |
| 18-30 y | 8 | 11.94% | 40* | 59.70% |
| 31-50 y | 29* | 43.29% | 19 | 28.36% |
| 51-70 y | 26 | 38.80% | 7 | 10.45% |
| >71 y | 4 | 5.98% | 1 | 1.49% |
| Total | 67 | 100% | 67 | 100% |
| χ2: 36.1561, *p-Value: 0.00001 (Statistically significant) | ||||
Overall gender distribution
In the gender-wise distribution of the study population, we were found that amongst the study population, there was predominance of male participants on ATT with ADD (n=44, 65.68%) and on ATT (n=40, 59.70%) as compared to female participants on ATT with ADD (n=23, 34.33%) and on ATT (n=27, 40.30%) (fig. 1)
Statistical analysis with Chi-square test gives χ2: 0.5105 with p-Value: 0.474933 suggesting no statistical significance.
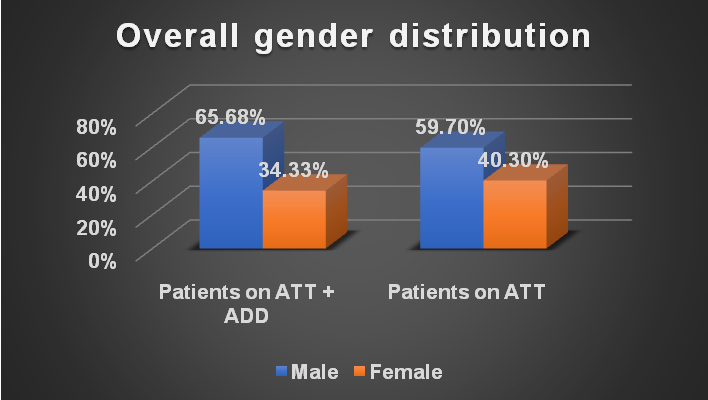
Fig. 1: Overall gender distribution (n=134)
Overall weight distribution
In the weight-wise distribution of the study population, we were found that maximum number of the patients in the study belonged to the weight group ≥35 to<50 kg. mean weight of the patients on ATT+ADD was 50.12 kg with SD 10.61 kg, which is higher than the mean weight of the patients on ATT (42.26 kg with SD 9.50 kg) (table 2)
Statistical analysis with Chi-square test gives χ2: 9.571 with p-Value: 0.0225 suggesting high statistical significance.
Table 2: Overall weight distribution (n=134)
| Weight | Patients on ATT+ADD | Patients on ATT | ||
| n | % | n | % | |
| <35 kg | 4 | 5.97% | 9 | 13.44% |
| ≥35 to<50 kg | 28* | 41.79% | 40* | 59.70% |
| ≥50 to<65 kg | 28* | 41.79% | 15 | 22.38% |
| ≥65 kg | 7 | 10.45% | 3 | 4.47% |
| Total | 67 | 100% | 67 | 100% |
| χ2: 09.571, *p-Value: 0.0225 (Statistically significant) | ||||
Overall diabetic distribution
In the diabetic status-wise distribution of the study population, we were found that amongst the study population, there was predominance of newly diagnosed DM with TB (n=36, 53.73%) as compared to existing DM with TB (n=31, 46.27%) (table 3).
Overall type of diabetes mellitus
In the type of diabetes mellitus-wise distribution of the study population, we were found that amongst the study population, there was predominance of type II DM (n=58, 86.57%) as compared to type I DM (n=9, 13.44%) (fig. 2).
Table 3: Overall diabetic distribution (n=67)
| Status | n | % |
| TB with Existing DM | 31 | 46.27% |
| TB with Newly Diagnosed DM | 36 | 53.73% |
| Total | 67 | 100% |
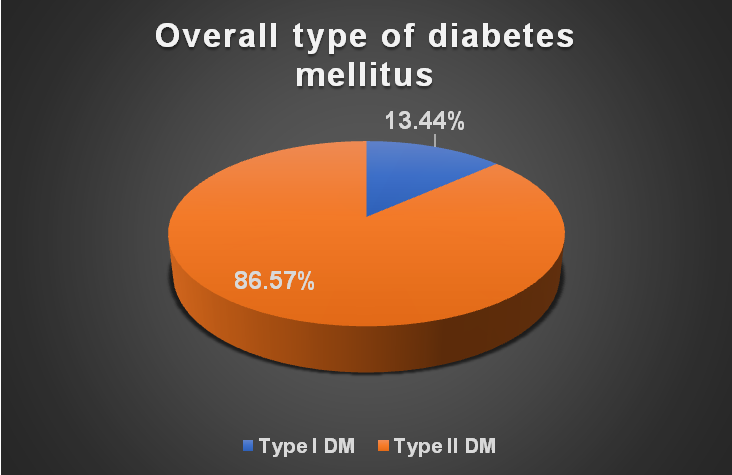
Fig. 2: Overall type of diabetes mellitus (n=67)
Correlation of ADR with patients on ATT+ADD and patient on ATT
In the correlation of ADR with patients on ATT+ADD and patients on ATT in study population, we were found that among the patients on ATT+ADD, 22 (32.84%) had adverse drug reactions and patients on ATT 6 (8.95%) had adverse drug reactions whereas 45 (67.17%) patients on ATT+ADD and 61 (91.04%) patients on ATT had no ADR (table 4).
Statistical analysis with Chi-square test gives χ2: 11.558 with p-Value: 0.00675 suggesting a statistically significant (higher rate of ADR in ATT+ADD patient).
Association of ADR in patients on ATT+ADD and patients on ATT only: (table 5)
In the association of patients on ATT+ADD with peripheral neuropathy of study population, we were found that the occurrence of peripheral neuropathy was higher in the ATT+ADD patients (N=8, 11.94%) as compared to the patients on ATT (N=2, 2.98%) alone.
In the association of patients on ATT+ADD with hepatitis (hepatotoxicity) of study population, we were found that the occurrence of hepatitis was higher in the ATT+ADD patients (N=7, 10.45%) as compared to the patients on ATT (N=1, 1.49%).
In the association of patients on ATT+ADD with hypoglycaemia of study population, we were found that among the patients on ATT+ADD, 2 (2.98%) had hypoglycaemia. Among the patients on ATT, no patient had hypoglycaemia.
*No statistical test was applied due to 0 subjects in the cells.
In the association of patients on ATT+ADD with renal failure of study population, we were found that only one patient suffered from renal failure and the patient was receiving on ATT only.
*No statistical test was applied due to 0 subjects in the cells.
In the association of patients on ATT+ADD with nausea/vomiting of study population, we were found that the occurrence of nausea/vomiting was higher in the ATT+ADD patients (N=10, 14.92%) as compared to the patients on ATT (N=3,4.47%).
In the association of patients on ATT+ADD with allergic reaction of study population, we were found only one patient suffered from allergic reaction and the patient was receiving on ATT only.
*No statistical test was applied-due to 0 subjects in the cells.
Table 4: Correlation of ADR with patients on ATT plus ADD and patients on ATT (n=134)
| ADR | Patients on ATT+ADD | Patients on ATT | ||
| n | % | n | % | |
| Yes | 22* | 32.84% | 6 | 8.95% |
| No | 45 | 67.17% | 61 | 91.04% |
| Total | 67 | 100% | 67 | 100.00% |
| χ2: 11.558, *p-Value: 0.00675 (Statistically significant) | ||||
Table 5: Association of ADR in patients on ATT+ADD and patients on ATT (n=134)
| ADR | Patients on ATT+ADD | Patients on ATT | ||
| Yes n (%) | No n (%) | Yes n (%) | No n (%) | |
| Peripheral neuropathy | 8 (11.94%) | 59 (88.05%) | 2 (2.98%) | 65 (97.01%) |
| Hepatitis (Hepatotoxicity) | 7 (10.45%) | 60 (89.55%) | 1 (1.49%) | 66 (98.50%) |
| Hypoglycaemia | 2 (2.98%) | 65 (97.01%) | 0 (0%) | 67 (100%) |
| Renal failure | 0 (0%) | 67 (100%) | 1 (1.49%) | 66 (98.50%) |
| Nausea/vomiting | 10 (14.92%) | 57 (85.07%) | 3 (4.47%) | 64 (95.52%) |
| Allergic reaction | 0 (0%) | 67 (100%) | 1 (1.49%) | 66 (98.50%) |
| Total | 67 | 100% | 67 | 100% |
DISCUSSION
An 18 mo prospective observational study was conducted in patients taking ATT+ADD and patients taking ATT alone and being registered under NTEP in the tertiary care teaching hospital from March 2021 to September 2022. In India, there is a concurrent increase in coexistence of diabetes in tuberculosis patients [1].
Recently numbers of studies reported from diabetes were at threefold higher risk of developing Tuberculosis. Apart that, studies also revealed that subjects screened for Diabetes among Tuberculosis patients reported a increased risk of Diabetes incidence among Tuberculosis patients ranging from 1.9% to as high as 35% [4].
ATT+ADD outcome and demography
The present study included newly diagnosed and existing diabetic patients with tuberculosis.
Analysis of patients receiving ATT and ADD revealed that the maximum number of patients were age group of 31-50 y which in accordance to the study of Balakrishnan et al. (2012) [1], Kumar et al. (2013) [5] and Hye E et al. (2017) [6] were reported highest incidence of DM in the age group of older than 30 y. In present study DM were more in males as compared to females (65.68% vs 34.33%) which is similar to above studies [1, 4–6]. Our results showed that patients receiving ATT+ADD were more likely to be male and of older age group while patients having compared to patients receiving ATT only. In diabetic patients the risk of TB appears to increase with age.
The present study revealed male sex and DM to be risk factors for sputum culture positivity after two months of anti-TB treatment in analysis. Also, it was found in this study that maximum number of ATT+ADD patients were in the weight range of 35-50 kg for both groups compared to other studies [1, 4-6].
ATT+ADD outcome and prevalence
In the summarised result, we can observe that prevalence rate of DM is 20.74% among TB patients. This is higher as compared to following described studies.
In previous literature a wide prevalence of 11.5% of diabetes in Patel et al. (2021) study, 12.4% of diabetes in Debashish Deyet et al. (2015) study, 13% of diabetes in Kumar et al. (2013) has been reported in active TB patients [4, 5, 7].
Studies of Huber FG et al. (2022) has reported 5% of newly diagnosed diabetes among TB patients [8]. Our findings are compare with lower incidence those of a study conducted in Indonesia that reported a prevalence of DM of 29.6% in patients with TB [9]. Similar findings were reported in Malaysia: DM prevalence was 26.7% in patients with TB [6].
ADR and Antitubercular treatment with antidiabetic drugs
Our study finding revealed that most common ADR in patient receiving ATT+ADD was nausea/vomiting (14.92%) followed by peripheral neuropathy (11.94%) as compared to patients receiving ATT only. On the causality assessment by WHO scale, most ADRs were classified as “Possible” among both groups. Early management of side effects of anti-TB drugs along with strict control of diabetes is necessary for management of patients of TB and DM [10].
S Patil et al. (2020) reported that the most common ADR in diabetic tuberculosis patients was gastritis (75%), followed by peripheral neuropathy (68%) and vomiting (45%). Gastritis was also the most commonly reported side effect in non-diabetic tuberculosis patients (85%), followed by vomiting in 65%. Impaired response in diabetes to MTB infection is associated with poor glucose control. Molecular studies show that mutation in MTB Kat G gene, which protect the bacteria against oxidative stress but encodes a catalase peroxidase which transform isoniazid into its active form can lead to drug resistance. Strains with these mutations may be better able to thrive in pts with type 2 DM in whom production of ROS is impaired. Other mechanisms in DM include failure of phagocytosis and inadequate T cell response [10].
Finding from study by Marra F et al. (2007) suggests that a large number of ADRs were observed. The nature of these events (most were hepatic, gastrointestinal or skin-related) which occurred within the first 100 days with ATT alone [11]. Yee et al. (2003) have reported the incidence of serious ADRs to first-line ATT. In their study of 430 patients, 46 serious events occurred involving 37 patients (incidence of 1.48 per 100 person-months) [12]. Singh A et al. (2017) and Jones l et al.(2022) study reported ADR like hypoglycaemia, gastrointestinal system, epigastric pain, skin and appendages, musculoskeletal, cardiovascular, respiratory system occurred with ADD [13, 14].
The present study reported the adverse drug reactions due to simultaneous use of anti-tubercular drugs with anti-diabetic drugs as of ATT interacts with ADD, which may lead to hyperglycemia, nephrotoxicity, reduced therapeutic effect and plasma concentration of drug which can cause poor compliance to antitubercular treatment with antidiabetic drugs. Also, ATT and ADD alone can cause adverse drug reactions.
CONCLUSION
This study emphasizes that basic information regarding detailed demographic dataand all newly diagnosed tuberculosis patients must be screened for diabetes mellitus and existing diabetes mellitus patients should be treated effectively for better management of tuberculosis with diabetes mellitus.
Drug-drug interactions adversely affect the plasma concentration of concomitant drug as well as reduces the therapeutic effect of drugs. That’s why dose modification and indication-based prescription is needed for the prevention of drug-drug interaction and adverse drug reactions to enhance drug safety.
ACKNOWLEDGEMENT
We would like to dedicate this work to the respected Dean of Government Medical College Surat, Dr. Ritambhara Mehta; Professor and Head of the Pharmacology Department, Government Medical College Surat, Dr. Chetankumar R. Acharya; Professor and Head of the Respiratory Medicine Department, Government Medical College Surat, Dr. Parul Vadgama; and patients of the Respiratory Medicine Department, New Civil Hospital, Surat.
FUNDING
Nil
LIMITATION
The study has some strengths and limitation; the patients were not actively reached on self-report. The serum level of antitubercular treatment and antidiabetic drugs is not measured in the institute so the drug-drug interactions were not measured for simultaneous use of both treatments.
AUTHORS CONTRIBUTIONS
Concept and design: Dr. Jeenal Mistry, Dr. Anita Sinha, Acquisition, analysis, or interpretation of data: Dr. Jeenal Mistry, Dr. Anita Sinha, Dr. B. Divakar, Dr. Mrugank Patel, Dr. Nayan Gavli, Drafting of the manuscript: Dr. Jeenal Mistry, Dr. Mrugank Patel, Dr. Nayan Gavli, Critical review of the manuscript for important intellectual content: Dr. Jeenal Mistry, Dr. Anita Sinha, Dr. B. Divakar, Supervision: Dr. Jeenal Mistry, Dr. Nayan Gavli.
CONFLICTS OF INTERESTS
Declared none
REFERENCES
Balakrishnan S, Vijayan S, Nair S, Subramoniapillai J, Mrithyunjayan S, Wilson N. High diabetes prevalence among tuberculosis cases in Kerala, India. PLOS One. 2012;7(10):e46502. doi: 10.1371/journal.pone.0046502, PMID 23077512.
Harsh V, Aswinikumar C, Sudan P, Sanjeeva K, Sheel V, Sachdeva K. India TB report national tuberculosis 2020. Central TB Division, Ministry of Health and Family Welfare; 2020. p. 1-266.
Shewade HD, Jeyashree K, Mahajan P, Shah AN, Kirubakaran R, Rao R. Effect of glycemic control and type of diabetes treatment on unsuccessful TB treatment outcomes among people with TB-diabetes: a systematic review. PLoS One. 2017;12(10):e0186697. doi: 10.1371/journal.pone.0186697, PMID 29059214.
Dey D, Patra BC, Ghosh D, Bengal W, Bengal W. High diabetes prevalence among males tuberculosis patients in East Medinipur District of West Bengal, India. 2015;3(4):940-4.
India Tuberculosis Diabetes Study Group. Screening of patients with tuberculosis for diabetes mellitus in India. Trop Med Int Health. 2013;18(5):636-45. doi: 10.1111/tmi.12084.
Hye E, Jung L, Lee M, Ae Y, Ah K, Leem Y. Prevalence and impact of diabetes mellitus among patients with active pulmonary tuberculosis in South Korea. Lung. 2017 Apr;195(2):209-15. doi: 10.1007/s00408-017-9978-4.
Patel N, Jain S, Khirid S, Sharma R. Prevalence of diabetes mellitus among pulmonary TB patients in a Tertiary Care Hospital of Ahmedabad city, Gujarat. Healthline. 2021;12(2):49-53. doi: 10.51957/Healthline_200_2020.
Huber FG, Kristensen KL, Holden IK, Andersen PH, Bakir B, Jorgensen A. The prevalence of diabetes among tuberculosis patients in denmark. BMC Infect Dis. 2022;22(1):64. doi: 10.1186/s12879-022-07048-4, PMID 35045811.
Alisjahbana B, Van Crevel R, Sahiratmadja E, Den Heijer M, Maya A, Istriana E. Diabetes mellitus is strongly associated with tuberculosis in Indonesia. Int J Tuberc Lung Dis. 2006;10(6):696–700. Available from: https://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed10&NEWS=N&AN=43845033.
Patil S. Adverse drug reactions of anti-tubercular medications in Mdr and Xdr Tb patients with diabetes mellitus. Chest. 2020;157(6):A59. doi: 10.1016/j.chest.2020.05.068.
Marra F, Marra CA, Bruchet N, Richardson K, Moadebi S, Elwood RK. Adverse drug reactions associated with first-line anti-tuberculosis drug regimens. Int J Tuberc Lung Dis. 2007;11(8):868-75. PMID 17705952.
Yee D, Valiquette C, Pelletier M, Parisien I, Rocher I, Menzies D. Incidence of serious side effects from first-line antituberculosis drugs among patients treated for active tuberculosis. Am J Respir Crit Care Med. 2003;167(11):1472-7. doi: 10.1164/rccm.200206-626OC, PMID 12569078.
Singh A, Dwivedi S. Study of adverse drug reactions in patients with diabetes attending a tertiary care hospital in New Delhi, India. J Dent Educ [Internet]. 2012;76(11):1532–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23144490.
Jones L, Jones AM. Suspected adverse drug reactions of the type 2 antidiabetic drug class dipeptidyl peptidase IV inhibitors (DPP4i): can polypharmacology help explain? Pharmacol Res Perspect. 2022;10(6):e01029. doi: 10.1002/prp2.1029, PMID 36468400.