
Int J Curr Pharm Res, Vol 17, Issue 6, 30-35Original Article
ANTIDEPRESSANT ACTIVITY OF PERICARPIUM GRANATI PEELS IN ALBINO MICE-A BENEFICIAL HERBAL TREATMENT STRATEGY
AMAN SHARMA1*, AJEET PAL SINGH1, AMAR PAL SINGH1, MAMTA KUMARI2
1Department of Pharmacology, St. Soldier Institute of Pharmacy, Lidhran Campus, Behind NIT (R. E. C.), Jalandhar–Amritsar by pass, NH-1, Jalandhar-144011, Punjab, India. 2Department of Pharmacology, JSS College of Pharmacy, JSS Academy of Higher Education and Research, Ooty, The Nilgiris, Tamil Nadu, India
*Corresponding author: Aman Sharma; *Email: aman.shamaas78@gmail.com
Received: 12 Aug 2025, Revised and Accepted: 02 Oct 2025
ABSTRACT
Objective: To evaluate Antidepressant Activity of ethanolic and aqueous peel extracts of Pericarpium Granatiin albino mice by using Forcedswim test (FST), Open field test (OFT) and tail suspension test (TST)) parameters.
Methods: For the study, 25–40 g adult Laca albino mice of either sex, age between 4-5 mo procuredfrom Lala Lajpat Rai University of veterinary and animal sciences, Hisar (125004),India and were housed in polypropylene cage. They were maintained under standard laboratoryconditions (temperature 25±2º C with 12/12h night/dark cycle), were fed with standard pelletdiet and water ad libitum.
Results: To evaluate the pharmacological effectiveness of various successive extracts of Pericarpium granati in Laca albino mice was investigatedin fourdifferent groups; Naive Control, Standard (Imipramine 10 mg/kg), Test Group-І, 200 mg/kg p. o. Pericarpium granati ethanolic extracts, Test Group-ІІ, 200 mg/kg p. o. Pericarpium granatiaqueous extracts. The numbers of parameters in relation to the depression were observed and thesignificant (P<0.01) decreased in the duration of immobility time were observed in Test Group-І(200 mg/kg) as compared to control group.
Conclusion: This study clearly demonstrated that ethanolic extract of Pericarpium granati might serve as apowerful antidepressant agent in adult Laca albino mice without causing any serious toxiceffects.
Keywords: Antidepressant activity, Pericarpium granati, Neurological disorder, Blood glucose, Glucosetolerance
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijcpr.2025v17i6.7064 Journal homepage: https://innovareacademics.in/journals/index.php/ijcpr
INTRODUCTION
Neuropsychiatric disorder mainly schizophrenia, depression, bipolar disorder affecting millions of people around the world. According to WHO, depression is expected to become the second leading cause of disease related disability by the year 2020, following heart disease [1]. Depression is one of the most common neuropsychiatric disorders characterized by low mood and aversion to activity that can affect person’s thoughts, behavior, feelings and sense of wellbeing [2, 3]. It is highly heritable, with roughly 40–50% of the risk for depression being genetic, although the specific genes that underlie this risk have not yet been identified. The remaining50–60% of the non-genetic risk also remains poorly defined, with suggestions that early childhood trauma, emotional stress, physical illness, and even viral infections might be involved [4]. The primary symptoms of depression involved depressed mood, low self-esteem, guilt, difficulty in concentration, suicidal ideation, thoughts of death etc. Based on various pharmacological evidences, it is believed that, each neurotransmitters exhibit particular effect on depressive behavior [5, 6]. Pharmacotherapy for depression has been involved effects on noradrenergic and serotonergic systems. The inability of these antidepressant agents to provide an adequate explanation for the shortcomings such as low remission/high treatment-resistance rates, treatment failures, slow onset of action, side effects [7, 8]. However, the administration of natural compounds to prevent, slow down and reverse the occurrence of depression [9]. Because of the higher safety, low toxicity, antioxidant properties, minimum cost and multiple benefits the natural products attaining great attention in the prevention of depression and other psychiatric disorders [10]. Pericarpium Granati belonging to family Lythraceae is a magical herb acts as a powerful antioxidant and remedial agent for the management of various disorders.[11] The plant possesses wide range of biological activities in several diseases like cancer, arthritis, urinary infections, digestive disorders, skin disorders, etc. [12] Additionally, the plant also exhibits potentnephroprotective, hepatoprotective, analgesic and anti-inflammatory effects[13-14] The main components responsible for biological activity of Pericarpium Granati are polyphenols, flavonoids, anthocyanins, fatty acids, alkaloids, vitamins, sugars and organic acids. Due to the breakdown of flavonoids sugar complexes during fermentation process, the end products contain high concentrations of free polyphenols, which results in potent biological activity [15]. Therefore, the study is based on the impact of flavonoid rich plant in the management of depression. Additionally, there are very few reports, which reflect the medicinal effectiveness of the peel part of Pericarpium Granati. Peel is the important nonedible part of fruit, which makes40% of the total mass of the fruit, and is usually considered as a waste. However, peels also contain phytochemicals that are medically and nutritionally important for pharmacological activity [16, 17]. Recently, Pomegranate peels extracts have attracted more attention because of their potential use as natural food preservatives and nutraceuticals [18]. Available evidences revealed that Pomegranate fruit and juice helps in improvement of blood pressure, blood glucose, and cholesterol levels [19, 20]. Also, the red peels have more antioxidant property than the juice, in several instances [21]. Although the beneficial effects of pomegranate peels extract still remains unclear in various experimental and clinical studies. For that reason, to understand the effectiveness, the present work designed to evaluate the antidepressant effect of the ethanolic and aqueous peel extracts of Pericarpium Granati in the models predictive of antidepressant activity.
MATERIALS AND METHODS
Instruments
Actophotometer, pH meter, glucometer, digital balance.
Drugs and chemicals
The described experiments used chemicals and drugs such as ethanol and Imipramine hydrochloride. The plant peels were collected from local area of Jalandhar, Punjab in the month of November, 2020. The peels were dried, powdered and subjected to extraction. Both extracts (ethanolic and aqueous) of Pericarpium granati peels were authenticated by Dr. Sunita Garg, Emeritus Scientist, CSIR-NISCAIR, Delhi, India (Ref. No. SSIP/Pharm/2894-C/2020). The sample of ethanolic and aqueous extracts of Pericarpium granati peels and standard drug were prepared by dissolving in deionized water at the doses of 200 mg/kg for sample extract and 10 mg/kg for standard drug respectively.
Animals
Healthy adult Laca albino mice of either sex, age between 4-5 mo weighing 25-40g, procured from Lala Lajpat Rai University of veterinary and animal sciences, Hisar (125004), India and were housed in polypropylene cage. They were maintained under standard laboratory conditions (temperature 25±2º C with 12/12h night/dark cycle), were fed with standard pellet diet and water ad libitum. The study protocol was approved by the Institutional Animal Ethics Committee (IAEC) [Protocol no.: IAEC/SSIP/2020/PR-011] of the Institute under the guideline of Committee for the Purpose of Control and Supervision on Experiments on Animals(CPCSEA), Ministry of Environment and Forests, New Delhi. All the ethical protocols were followed the ARRIVE guidelines, and the national institutes of health guide for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978).
Acute toxicity study
The animal acute toxicity was carried out as per the guideline set by OECD 425 and animals were observed for mortality and behavioral changes [22, 23].
Experimental design
The behavioral effects of orally administered Pericarpium granati peels (200 mg/kg) ethanolic and aqueous extracts will be evaluated in mice with forced swim test (FST), Tail Suspension test (TST) and Open Field Test (OFT). The effects of standard drug Imipramine 10 mg/kg will also be assessed. The animals are divided into four different groups and each group contains six Lacaalbino mice.
Table 1: Experimental design for the study
| Grouping of animals | |
| Groups | Treatment |
| Group-I(Control group) | Naïve animal, received standard pellet diet and tap water ad libitum daily. |
| Group-II(Standard group) | Standard group received imipramine 10 mg/kg orally daily |
| Group-III(Test group-I) | Testgroup-Ireceived200 mg/kg ethanolic extract orally daily |
| Group-IV(Test group-II) | Test group-II received 200 mg/kg aqueous extract orally daily |
Forced swimming test
The modified forced swimming test was designed and used in accordance with the procedures described. Mice were taken and placed individually in a cylinder (50 × 20 cm) filled to a depth of 30 cm, by fresh water (22±2 °C). The climbing, swimming and immobility behaviors were recorded during the 6 min for 14 d. Two swim sessions were conducted: a 15 min pretest, followed 24 h later by a 6 min test for the evaluation total immobility time [24, 25]. Tests were done on 0th day and 14th day of the study to evaluate chronic effect of test drugs.
Tail suspension test in mice
The TST shares a common theoretical basis and behavioral measure with the FST. In this, mice both acoustically and visually isolated were suspended above the floor by adhesive tape placed approximately 1 cm from the tip of the tail. The remained immobile time of TST was quantified for 6 min. Mice were considered immobile only when they hung passively and completely motionless [26]. The test was conducted on 0th and 14th day of protocol.
Open field test
To evaluate the effect of the drugs on spontaneous locomotor activity, mice were individually placed in an opaque Plexiglas cage (61´ 61 cm) with walls 40 cm height. Entire apparatus was painted black except 3 mm white lines that divided the floor into 16 equalsizes square. The apparatus was illuminated with a low intensity diffuse light (45W) situated 45 cm above the floor. When the hind legs crossed the line of the squares, the mice was considered to have crossed from one square to another (crossing). After each mice was tested in the OFT, the cage was carefully cleaned with a solution containing 0.5% ammonia, 15% ethanolic, 10% extra, 5% isopropanol and 59.5% purified water to remove the scent of the previously evaluated mice, which could modify the spontaneous behavior of the mice [27, 28]. Test was repeated on 0th and 14th day of experiment.
Biochemical estimation
On 15th d, blood (0.3 ml) was withdrawn by puncturing retro-orbital plexus from all groups of mice. Blood samples were taken and the estimation of glucose was performed using Accu-Chek Active strip glucometer.
Fasting blood glucose
The blood glucose will measure by tail prick method using OneTouch glucose strips. Oral gavage of glucose solution 2 g/kg (40% aqueous solution) will be given to test animals. After glucose administration, glucose levels will be measured at 30, 60, 90and 120 min (Aguilar EC, Queiroz MDGMN, Oliveira DAD, Oliveira NJFD) [29]. After, the completion of experiments, animals were kept separately for 30 d under veterinary care till it completely recovers from stress and then return to animal housing room.
Statistical analysis
Results of behavioral and biochemical estimations have been indicated in terms of mean±SEM. The difference among means have been analyzed by One way ANOVA by using Dunnett’s multiple comparison test and Two way ANOVA by using Bonferroni post-tests. Level of significance was fixed at p<0.05, p<0.01 and p<0.001 accordingly. All the statistical analysis of experimental data will be performed on GraphPad Prism 5.00 software.
RESULTS
Preliminary phytochemical screening
The preliminary phytochemical analysis of ethanolic and aqueous extracts of Pericarpiumgranati peels showed the presence carbohydrates, proteins, amino acids, flavonoids, steroids, saponins, tannins and phenolic compounds.
Acute oral toxicity study
The ethanolic and aqueous extracts of Pericarpium granati peels showed neither behavioral changes nor mortality at dose 2000 mg/kg observed from 4 h to 14 d of toxicity study.
Evaluation of antidepressant activity
In present study, we selected different animal behavioral depressant models to produce a condition similar to human depression.
Effect of pericarpium granati peel extracts on FST model
In the present study, all the animals of different groups were treated for 7 d. The changes in immobility duration were studied after administrating drugs in separate groups of animals. The immobility parameter was recorded after 24 h and after 14 d. The duration of immobility significantly increased (P<0.05) in control group compared with the treatment groups. All the treatment groups showed significant decrease in the decrease in duration of immobility, when compared with control group. The test group-I showed highly effective results and it was near to the standard group.
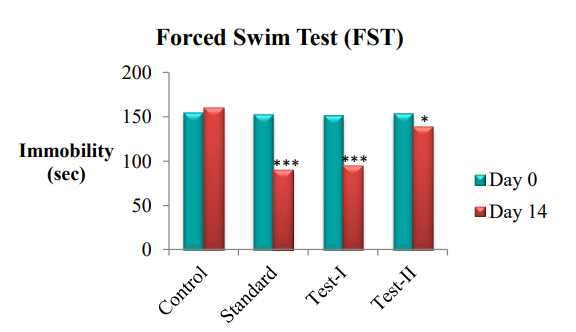
Fig. 1: Graphical representation of antidepressant effect of ethanolic and aqueous extracts of pericarpium granati peels on Forced Swim Test (FST), Value are represented as mean±SEM; n =6; *P<0.05, **P<0.01, ***P<0.001 indicate significant differences compared with the control group (Day 14)
Effect of Pericarpium granati peel extracts on TST model
The immobility time in the TST using mice was markedly reduced after treatment with Pericarpium granati Peels extracts and imipramine. There was significant (P<0.05) reductionin immobility time compared to vehicle treated group was observed. The test group-I showed significant (P<0.05) reduction in the duration of immobility. However, the Test group-II was less effective to reduce the duration of immobility.
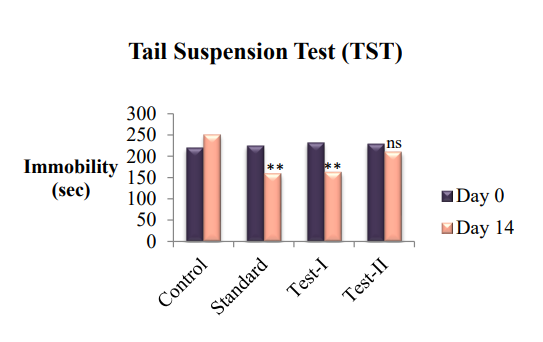
Fig. 2: Graphical representation of antidepressant effect of ethanolic and aqueous extracts of Pericarpium granati peels on Tail suspension Test (TST), value are represented as mean±SEM; n =6; *P<0.05, **P<0.01, ***P<0.001 indicate significant differences compared with the control group (Day 14) and ns indicate non-significant
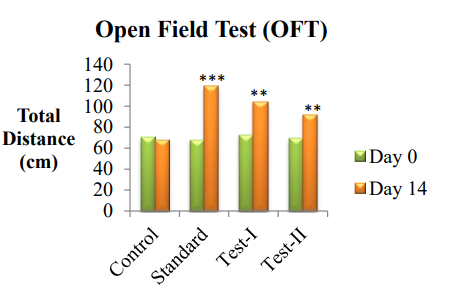
Fig. 3: Graphical representation of antidepressant effect of ethanolic and aqueous extracts of Pericarpium granati peels on open field test (OFT), value are represented as mean±SEM; n=6; *P<0.05, **P<0.01, ***P<0.001 indicate significant differences compared with the control group (Day 14)
Effect of Pericarpium granati peel extracts on OFT model
The total distance was recorded after 24 h and after 14 d in all the groups. Treatment and imipramine treated mice showed significant increase (P<0.05) in the number of rearings and number of squares crossed (P<0.01) as compared to control groups. Test group-I showed significant increase in the number of rearings (P<0.05) and number of squares crossed (P<0.01) near to the standard group. Additionally, Test group-II also showed increased number of rearings and square crossed as compared to control group.
Biochemical estimation
On the last day of the study, blood was collected from all animal by puncturing retro-orbital plexus and blood samples were used for the biochemical analysis of glucose levels in the depressant mice models. Animals of control group observed with low utilization of glucose and the level of glucose is high. The glucose level significantly reduced (P<0.05) in treatment groups compared with the control group. All the treatment groups showed significant glucose utilization, when compared with the control group.
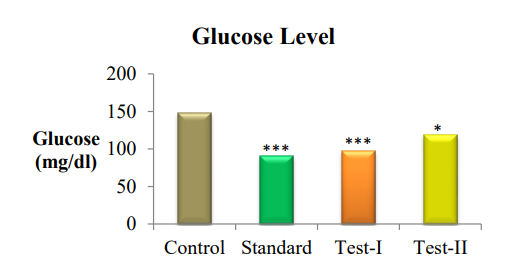
Fig. 4: Graphical representation of antidepressant effect of ethanolic and aqueous extracts of Pericarpium granati peels on Glucose level, value are represented as mean±SEM; n =6; *P<0.05, **P<0.01, ***P<0.001 indicate significant differences compared with the control group
DISCUSSION
Suffering from psychiatric diseases like depression, anxiety etc. Sometimes these mental illnesses are related with suicide attempts. In this regard, antidepressants in different categories are used but these drugs show pharmacological activity only in 75-80% of patients with different side effects [30]. Associated with antidepressant, researcher shifted their interest in discovering herbs with antidepressant-like activity. Several studies revealed that natural phenol rich compounds can decrease the progression of various neurological diseases. The phenolic compounds might contribute to the potent antidepressant and antioxidant activity [31, 32]. Based on the best available evidence, Pomegranate extract improves a depressive state and bone properties in menopausal syndrome model mice [12, 13]. Besides that, it was demonstrated that pomegranate exhibits low toxicity, no lethality, and well tolerated, when administered to mice [14, 15]. Currently, there are no reports demonstrating the direct action of pomegranate extract on the serotonergic system [33]. A study relates the antidepressant action of Pomegranate with polyphenols as their major active component, revealed that pomegranate extract induces an antidepressant-like effect by inhibiting brain MAO-B and MAO-A enzyme activity. They claimed that inhibition of MAO enzyme activity directly block 5-HT degradation and, therefore, show potent antidepressant activity [34]. In the present study, the efforts were made to clarify the possible mechanism of action that Pericarpium granati peels has been claimed traditionally in the treatment of the depression. FST is based on the assumption that when mice placed in a container filled with water, it will try to escape but eventually will exhibit immobility that may be considered a measure of behavioral despair [35]. The total immobility time in mice treated with the different extracts of Pericarpiumgranati peels was significantly reduced on day 14 compared with day 0 by performing FST and TST. Additionally, the extract treatment also increased struggling behavior in the FST and TST after treatment. Available findings give a clear idea about the significant correlation between the clinical efficacy and potency of antidepressants in the FST and TST [36, 37]. OFT describes photophobic behavior, in which animal avoids the bright open spaces and prefers to stay in close spaces. The analysis of locomotor behavior therefore represents measure of crossings in OFT [38]. The results revealed the significant difference in locomotor behavior after treatment with different extracts. Test group-I and Test group-II, both observed with increased locomotor activity as compared to control animals. During stress conditions, the sympathetic nervous system (SNS) stimulate the release of catecholamine from the adrenal medulla, further which results in rise of the blood glucose level by obstructing insulin release [39, 40]. In behavioral study, we also found increase in blood glucose level due to stress and behavioral impairment [41]. It was observed that blood glucose level was rises during depression but it reduces towards normal in] So, to overcome the limitations the treatment groups with respect to control group. These behavioral effects were similar to the available reports with conventional antidepressant, such as tricyclic, monoamine oxidase inhibitors and selective serotonin reuptake inhibitors [42, 43]. Recent findings linked the oxidative stress with the pathophysiology of depression [44]. Therefore, it is also possible that the antioxidant nature of the ethanolic extract may contribute to its antidepressant-like effect. Therefore, the available findings suggested that the ethanolic extract dose 200 mg/kg of Pericarpium granati peels having the antidepressant effect. It showed a protective effect on depressant mice model without any toxic effect.
CONCLUSION
From this study, it can be concluded that ethanolic extract of Pericarpium granati peels possesses potent antidepressant effect. This effect might be related to inhibition of monoamine neurotransmitters uptake thus increasing their availability in the synapse. However, further experiments are needed to identify possible mechanisms of action for futuristic study. List of abbreviations
ACKNOWLEDGMENT
It’s our privilege to express the profound sense of gratitude and cordial thanks to our respected chairman Mr. Anil Chopra and Vice Chairperson Ms. Sangeeta Chopra, St. Soldier Educational Society, Jalandhar for providing the necessary facilities to complete this research work.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
All authors have contributed equally
CONFLICT OF INTERESTS
Declared none
REFERENCES
Das MC, Srinivasa Rao ASR, Karuna Sri G. Antidepressant activity of brahmi in albino mice. J Clin Diagn Res. 2014;8(3):35-7. doi: 10.7860/JCDR/2014/7482.4098, PMID 24783074.
Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34(1):13-25. doi: 10.1016/s0896-6273(02)00653-0, PMID 11931738.
Castagne V, Moser P, Porsolt RD. Behavioral assessment of antidepressant activity in rodents. Buccafusco JJ (ed). Methods of Behavior Analysis in Neuroscience. 2nd ed. Boca Raton (FL): CRC Press/Taylor & Francis; 2009.
Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci. 2006;7(2):137-51. doi: 10.1038/nrn1846, PMID 16429123.
Brigitta B. Pathophysiology of depression and mechanisms of treatment. Dialogues Clin Neurosci. 2002;4(1):7-20. doi: 10.31887/DCNS.2002.4.1/bbondy, PMID 22033824.
Biological Sciences Curriculum Study, National Institutes of Health. Information about mental illness and the brain. In: NIH curriculum Supplement Series. Bethesda National Institutes of Health; 2007.
Sansone RA, Sansone LA. Dysthymic disorder: forlorn and overlooked? Psychiatry (Edgmont). 2009;6(5):46-51. PMID 19724735.
Hilty DM, Leamon MH, Lim RF, Kelly RH, Hales RE. A review of bipolar disorder in adults. Psychiatry (Edgmont). 2006 Sep;3(9):43-55. PMID 20975827.
Liu L, Liu C, Wang Y, Wang P, Li Y, Li B. Herbal medicine for anxiety depression and insomnia. Curr Neuropharmacol. 2015;13(4):481-93. doi: 10.2174/1570159x1304150831122734, PMID 26412068.
Yeung KS, Hernandez M, Mao JJ, Haviland I, Gubili J. Herbal medicine for depression and anxiety: a systematic review with assessment of potential psycho-oncologic relevance. Phytother Res. 2018;32(5):865-91. doi: 10.1002/ptr.6033, PMID 29464801.
Park SY, Na CS, Jeong WC, Lee JC. A literature study of Pericarpium Granati and cortex Betulae Platyphyllae. The Journal of Korean Oriental Medical Ophthalmology and Otolaryngology and Dermatology. 2012;25(3):13-33. doi: 10.6114/jkood.2012.25.3.013.
Bhowmik D, Gopinath H, Kumar BP, Kumar K. Medicinal uses of Punica granatum and its health benefits. J Pharmacogn Phytochem. 2013;1(5):28-35.
Kaur G, Jabbar Z, Athar M, Alam MS. Punica granatum (pomegranate) flower extract possesses potent antioxidant activity and abrogates Fe-NTA induced hepatotoxicity in mice. Food Chem Toxicol. 2006;44(7):984-93. doi: 10.1016/j.fct.2005.12.001, PMID 16426722.
Riaz A, Khan RA. Behavioral effects of Citrus limon and Punica granatum combinations in rats. Metab Brain Dis. 2017;32(1):123-31. doi: 10.1007/s11011-016-9884-0, PMID 27510713.
Shaygannia E, Bahmani M, Zamanzad B, Rafieian Kopaei M. A review study on Punica granatum L. J Evid Based Complementary Altern Med. 2016;21(3):221-7. doi: 10.1177/2156587215598039, PMID 26232244.
Cam M, Icyer NC, Erdogan F. Pomegranate peel phenolics: microencapsulation storage stability and potential ingredient for functional food development. LWT Food Sci Technol. 2014;55(1):117-23. doi: 10.1016/j.lwt.2013.09.011.
Grabez M, Skrbic R, Stojiljkovic MP, Rudic Grujic V, Paunovic M, Arsic A. Beneficial effects of pomegranate peel extract on plasma lipid profile fatty acids levels and blood pressure in patients with diabetes mellitus type-2: a randomized double-blind placebo controlled study. J Funct Foods. 2020;64:103692. doi: 10.1016/j.jff.2019.103692.
Wang BS, Leu KL, Huang GJ, Yeh CF, Tai HM, Ho WY. Protective effects of an aqueous Pericarpium Granati extract against inflammatory damage in mice. J Funct Foods. 2014;9:183-91. doi: 10.1016/j.jff.2014.04.022.
Sahebkar A, Ferri C, Giorgini P, Bo S, Nachtigal P, Grassi D. Effects of pomegranate juice on blood pressure: a systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. 2017;115:149-61. doi: 10.1016/j.phrs.2016.11.018, PMID 27888156.
Suman M, Bhatnagar P. A review on proactive pomegranate one of the healthiest foods. Int J Chem Stud. 2019;7(3):189-94.
Ventura J, Alarcon Aguilar F, Roman Ramos R, Campos Sepulveda E, Reyes Vega ML, Daniel Boone Villa VD. Quality and antioxidant properties of a reduced sugar pomegranate juice jelly with an aqueous extract of pomegranate peels. Food Chem. 2013;136(1):109-15. doi: 10.1016/j.foodchem.2012.07.039, PMID 23017400.
Pahwa P, Goel RK. Absence of anticonvulsant activity in Asparagus adscendens Roxb. hydroethanolic root extract against acute pentylenetetrazol and maximal electroshock induced convulsion mice models. J Pharm Neg Results. 2016;7(1):25-8.
Toxicity–up AO. OECD guideline for testing of chemicals. Vol. 17. Paris, France: Organization for economic co-operation and development; 2001. p. 1.
Detke MJ, Johnson J, Lucki I. Acute and chronic antidepressant drug treatment in the rat forced swimming test model of depression. Exp Clin Psychopharmacol. 1997;5(2):107-12. doi: 10.1037//1064-1297.5.2.107, PMID 9234045.
Castagne V, Moser P, Roux S, Porsolt RD. Rodent models of depression: forced swim and tail suspension behavioral despair tests in rats and mice. Curr Protoc Pharmacol. 2010;49(1):5.8. doi: 10.1002/0471141755.ph0508s49, PMID 22294373.
Strekalova T, Spanagel R, Dolgov O, Bartsch D. Stress induced hyperlocomotion as a confounding factor in anxiety and depression models in mice. Behav Pharmacol. 2005;16(3):171-80. doi: 10.1097/00008877-200505000-00006, PMID 15864072.
Gutierrez Garcia AG, Contreras CM, Mendoza Lopez MR, Cruz Sanchez S, Garcia Barradas O, Rodriguez Landa JF. A single session of emotional stress produces anxiety in wistar rats. Behav Brain Res. 2006;167(1):30-5. doi: 10.1016/j.bbr.2005.08.011, PMID 16216347.
Ishola IO, Ochieng CO, Olayemi SO, Jimoh MO, Lawal SM. Potential of novel phytoecdysteroids isolated from Vitex doniana in the treatment depression: involvement of monoaminergic systems. Pharmacol Biochem Behav. 2014;127:90-100. doi: 10.1016/j.pbb.2014.11.005, PMID 25449355.
Patel SS, Mehta V, Changotra H, Udayabanu M. Depression mediates impaired glucose tolerance and cognitive dysfunction: a neuromodulatory role of rosiglitazone. Horm Behav. 2016;78:200-10. doi: 10.1016/j.yhbeh.2015.11.010, PMID 26631485.
Jahromy MH, Khakpour S, Khorgami Z. The antidepressant like effects of Punica granatum (Pomegranate) extract in mice. Chin Med. 2014;5:1-6. doi: 10.4236/cm.2014.51001.
Pauleti NN, Mello J, Siebert DA, Micke GA, De Albuquerque CA, Alberton MD. Characterisation of phenolic compounds of the ethyl acetate fraction from Tabernaemontana catharinensis and its potential antidepressant-like effect. Nat Prod Res. 2018;32(16):1987-90. doi: 10.1080/14786419.2017.1359167, PMID 28764559.
Ferreres F, Grosso C, Gil Izquierdo A, Valentao P, Andrade PB. Phenolic compounds from Jacaranda caroba (Vell.) A. DC: approaches to neurodegenerative disorders. Food Chem Toxicol. 2013;57:91-8. doi: 10.1016/j.fct.2013.03.012, PMID 23524314.
Valdes Sustaita B, Lopez Rubalcava C, Gonzalez Trujano ME, Garcia Viguera C, Estrada Camarena E. Aqueous extract of pomegranate alone or in combination with citalopram produces antidepressant-like effects in an animal model of menopause: participation of estrogen receptors. Int J Mol Sci. 2017;18(12):2643. doi: 10.3390/ijms18122643, PMID 29257042.
Naveen S, Siddalingaswamy M, Singsit D, Khanum F. Anti-depressive effect of polyphenols and omega-3 fatty acid from pomegranate peel and flax seed in mice exposed to chronic mild stress. Psychiatry Clin Neurosci. 2013;67(7):501-8. doi: 10.1111/pcn.12100, PMID 24152226.
Yankelevitch Yahav R, Franko M, Huly A, Doron R. The forced swim test as a model of depressive like behavior. J Vis Exp. 2015;(97):52587. doi: 10.3791/52587, PMID 25867960.
Pesarico AP, Birmann PT, Pinto R, Padilha NB, Lenardao EJ, Savegnago L. Short and long term repeated forced swim stress induce depressive like phenotype in mice: effectiveness of 3-[(4-chlorophenyl)selanyl]-1-methyl-1H-indole. Front Behav Neurosci. 2020;14:140. doi: 10.3389/fnbeh.2020.00140, PMID 33192355.
Shashikumara S, CP, Sibgatullah M. Evaluation of antidepressant activity of ethanolic extract of Alangium Salvifolium leaves in swiss albino mice. Biomed Pharmacol J. 2017;10(1):427-33. doi: 10.13005/bpj/1125.
Fuchs E, Fliugge G. Experimental animal models for the simulation of depression and anxiety. Dialogues Clin Neurosci. 2006;8(3):323-33. doi: 10.31887/DCNS.2006.8.3/efuchs, PMID 17117614.
Jansen AS, Van Nguyen XV, Karpitskiy V, Mettenleiter TC, Loewy AD. Central command neurons of the sympathetic nervous system: basis of the fight-or-flight response. Science. 1995;270(5236):644-6. doi: 10.1126/science.270.5236.644, PMID 7570024.
Lim SM, Park SH, Sharma N, Kim SS, Lee JR, Jung JS. Blood glucose regulation mechanism in depressive disorder animal model during hyperglycemic states. Brain Res Bull. 2016;124:116-22. doi: 10.1016/j.brainresbull.2016.03.014, PMID 27034116.
Cox DJ, Gonder Frederick L. Major developments in behavioral diabetes research. J Consult Clin Psychol. 1992;60(4):628-38. doi: 10.1037//0022-006x.60.4.628, PMID 1506511.
Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci. 2006;7(2):137-51. doi: 10.1038/nrn1846, PMID 16429123.
Al Harbi KS. Treatment resistant depression: therapeutic trends challenges and future directions. Patient Prefer Adherence. 2012;6:369-88. doi: 10.2147/PPA.S29716, PMID 22654508.
Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci. 2006;7(2):137-51. doi: 10.1038/nrn1846, PMID 16429123.