
Int J Curr Pharm Res, Vol 17, Issue 6, 40-47Original Article
STUDY TO INVESTIGATE THE ANXIOLYTIC EFFECT OF RUBIA CORDIFOLIA LINN IN MICE
ANKUSH CHAUHAN*, AJEET PAL SINGH, AMAR PAL SINGH
Department of Pharmacology, St. Soldier Institute of Pharmacy, Lidhran Campus, Behind NIT (R. E. C.), Jalandhar–Amritsar by pass, NH-1, Jalandhar-144011, Punjab, India
*Corresponding author: Ankush Chauhan; *Email: ankushchauhan611@gmail.com
Received: 01 Aug 2025, Revised and Accepted: 05 Oct 2025
ABSTRACT
Objective: To evaluate anxiolytic activity of extracts of Rubia cordifolia in albino mice by using Actophotometer (locomotor activity), Elevated Zero maze, Mirrored Chamber and biochemical estimation like estimation of plasma corticosterone levels.
Methods: Healthy, Swiss Albino mice weighing 20-35 g and aged 6-8 w were maintained under standard laboratory conditions with controlled temperature (25±2 °C), humidity (40±10 %) and 12 h light and dark cycles each. The animals were fed with standards rodent pellet diet and water ad libitum. Rubia cordifolia extract will be administered p. o.. to mice daily in two different doses (300 and 600 mg/kg/p. o.) for 14 d. All the animals will be evaluated for behavioral parameters, i. e. Actophotometer (locomotor activity), Elevated Zero maze, Mirrored Chamber and biochemical estimation like estimation of plasma corticosterone levels will be performed.
Results: In the present study, we have undertaken the anxiolytic activity of extracts of Rubia cordifolia in Swiss albino mice, at the dose of (300 and 600 mg/kg/p. o.) with oral administration respectively.
Conclusion: In conclusion extracts of Rubia cordifolia have shown significant results as anxiolytic activity using locomotor activity), Elevated Zero maze, Mirrored Chamber.
Keywords: Rubia Cordifolia, Anxiety, Locomotors activity, Plasma corticosterone, Elevated zero maze and mirrored chamber
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijcpr.2025v17i6.7072 Journal homepage: https://innovareacademics.in/journals/index.php/ijcpr
INTRODUCTION
Anxiety is a natural response to stress and can be helpful in certain circumstances. The word "anxiety" refers to a common emotion that people have in response to stress, danger, or threats. People who experience anxiety usually feel tense, unpleasant, and upset [1]. Excessive and unreasonable worry over tasks, situations, or even individual things is a hallmark of anxiety disorders. Worry disorders are not a single sickness, but rather a collection of conditions marked by excessive tension, discomfort, and persistently high levels of worry. The substantial prevalence of anxiety disorders in the global general population is demonstrated by recent epidemiological studies [2]. Anxiety disorders affect 13.3% of Americans, with an estimated 15.6 billion Americans experiencing anxiety problems annually [3]. When a person has anxiety to the point where it substantially disrupts their everyday life and prevents them from achieving their goals, they are likely to be diagnosed with an anxiety disorder. One in twenty persons suffers from an anxiety disorder, which is the most prevalent type of mental illness. According to epidemiological data, anxiety is a prevalent and significant issue in later life and has been linked to increased impairment risk in older persons overall [4].
If left untreated, anxiety disorders can cause significant distress and impair a person's life by influencing their thoughts, feelings, and behaviors. anxiety can usually be effectively treated. Women are at least twice as likely as males to experience one of the three anxiety disorders during their lifetime [5, 6]. Anxiety is a normal and necessary part of life. Evolutionarily speaking, it is crucial because people usually feel anxious when confronted with environmental dangers like coming across a lion, which is not a common worry in contemporary society, or when there is a shortage of food or other resources, or when they are accepted by their peers and society as a whole [7]. Anxiety is an adaptive emotion that helps people respond appropriately to stressful situations by changing their behavior and physiology. When faced with a stressful circumstance, everyone feels a certain level of anxiety and trepidation. Anxiety disorders can be defined as situations that fully disrupt a person's daily life. It has been proposed that cognitive biases have a role in the development and may even be the cause of anxiety. Restlessness, exhaustion, mental tension, irritability, sleep difficulties, poor stress management, chest discomfort, abdominal trouble, tachycardia, shortness of breath, shaking, blushing, diarrhea, sweating, and so on are some of the physical signs of anxiety disorders. Anxiety is a mental illness that manifests as excessive worry, motor tension, and exhaustion, along with involuntary symptoms like headache, palpitations, chest tightness, restlessness, and moderate stomach pain that may be triggered by a dangerous stimuli or circumstance [8, 9]. Indian madder, or Rubia cordifolia L. (Rubiaceae), is a significant medicinal plant that is used in Indian traditional medicine to treat a variety of illnesses. The climbing plant Rubia cordifolia grows in the Nilgiris, the North-West Himalayas, and other hilly regions of India at elevations between 1500 and 2500 meters. There are other colloquial names for plant drugs, including Majathi, Assamme Mandar, and Sanskrit Aruna. English Indian Madder in Malayalam, Bengali Manjith, Marathi Manjesta Manjit, Hindi Majit [10]. It is a climbing herb, perennial, or prostate. Sharp, 4-angled, minutely prickly stems; elliptic to ovate-cordate, long petiolate leaves in whorles of 6–8; greenish-yellow flowers in axillary panicles of dichotomous cymes; two-celled, globose, smooth, shining, purplish-black when ripe fruits June through October and Mainly the root and stem are used. Although practically every portion is used, the leaves, root barks, and fruits are crucial for making medications. In the indigenous medical system, Rubia cordifolia is utilized as medication; its fruits and barks are the most effective [11]. According to traditional folklore, it can help with weight control, skin care, diabetes management, cancer prevention, bone health, and inflammation reduction. Additionally, they are used to treat fungal infections, constipation, skin aging, sleep disorders, and stress [12]. The species can tolerate temperatures between 15 and 35 degrees Celsius at high elevations in the Himalayas. The ideal soil for the plant is light, loose, moist, and somewhat shaded. Because the root penetrates deeply into the soil, agriculture benefits from porous, well-aerated soils. In order to keep the beds moist, weekly irrigation is advised. Stem fragments are directly planted for vegetative propagation [13]. When compared to a group of albino mice that were given Aβ 25-35, the extract from Rubia cordifolia demonstrated a strong protective effect against neurodegeneration and an enhancement in memory retention ability. Due to the GSH and vitamin C content of the plant, Rubia cordifolia exhibits neuroprotective activity by preventing the reduction and increasing GSH levels by inducing GCLC (c-glutamylcysteine ligase) expression, reducing oxidative stress levels by direct scavenging, and decreasing iNOS expression.
MATERIALS AND METHODS
The research protocol of this study has been approved by Institutional Animal Ethics Committee (IAEC) of St. Soldier institute of Pharmacy, Jalandhar, Punjab vide approval no. 2011/PO/Re/S/18/CPCSEA and 1/5/2018 Protocol No. IAEC/SSIP/2020/PR-012.
Animals
Swiss Albino mice weighing 20-35 g and aged 6-8 w were procured from National Institute of Pharmaceutical Education and Research, Mohali, Punjab. The animals were acclimatized for seven days to the housing conditions of Central Animal House Facility of St. Soldier institute of Pharmacy, Jalandhar prior to experiments. Animal were housed and maintained under standard laboratory conditions with controlled temperature (25±2 °C), humidity (40±10 %) and 12 h light and dark cycles each. The animals were fed with standards rodent pellet diet and water ad libitum. The experiments were carried out between 09:00 to 17:00 h. The laboratory animals were maintained as per CPCSEA guidelines.
Drugs and chemicals
Rubia cordifolia extract was purchased from Kshipra biotech pvt. Ltd., Indore
Administration of Rubia cordifolia extract
Rubia cordifolia extract will be administered p. o. to mice daily in two different doses (300 and 600 mg/kg/p. o.) for 14 d. The feed and water consumption of the treated animals was monitored.
Experimental design
Treatment schedule
Rubia cordifolia extract will be administered p. o. to mice daily in two different doses (300 and 600 mg/kg/p. o.) for 14 d. All the animals will be evaluated for behavioral parameters, i. e. Actophotometer (locomotor activity), Elevated Zero maze, Mirrored Chamber and Biochemical estimation like estimation of plasma corticosterone levels will be performed.
Control group
Mice were handled gently without any stress and after 14 d all the animals will be evaluated for behavioral parameters, i. e. Actophotometer (locomotor activity), Elevated Zero maze, Mirrored Chamber, and Biochemical estimation like estimation of plasma corticosterone levels will be performed.
RCE (300 mg/kg (p. o.))
Rubia cordifolia extract 300 mg/kg (p. o.) were administered for 14 successive days. All the animals will be evaluated for behavioral parameters, i. e. Actophotometer (locomotors activity),. Elevated Zero maze, Mirrored Chamber and Biochemical estimation like estimation of plasma corticosterone levels will be performed.
RCE (600 mg/kg (p. o.))
Rubia cordifolia extract 600 mg/kg (p. o.) were administered for 14 successive days. All the animals will be evaluated for behavioral parameters, i. e. Actophotometer (locomotors activity), Elevated Zero maze, Mirrored Chamber, and Biochemical estimation like estimation of plasma corticosterone levels will be performed.
APZ (0.25 mg/kg i. p.)
Alprazolam 0.25 mg/kg i. p. was administered for 14 successive days. All the animals will be evaluated for behavioral parameters, i. e. Actophotometer (locomotor activity). Elevated Zero maze, Mirrored Chamber, and Biochemical estimation like estimation of plasma corticosterone levels will be performed.
Table 1: Treatment of different groups
| S. No. | Group | Treatment (For 14 d) | Behavioral and biochemical estimations |
| I | Normal control, normal saline 0.9% NaCl (10 ml/kg, or 1 ml of/100 g body weight p. o.) | Yes | Yes |
| II | RCE 300 mg/kg (p. o.) | Yes | Yes |
| III | RCE 600 mg/kg (p. o.) | Yes | Yes |
| IV | Alprazolam (0.25 mg/kg i. p) | Yes | Yes |
Behavioral estimations
Locomotors activity
The majority of medications that work on the central nervous system affect how humans and animals move. Alcohol and barbiturates are examples of CNS depressants that decrease motor function. Stated differently, locomotor activity may serve as a gauge of mental activity's alertness or wakefulness. An actophotometer, which uses photoelectric cells that are coupled in circuit with a counter, makes it simple to quantify locomotor activity. A count is made when the animal cuts off the light beam that is hitting the photocell. The animal's movement could be recorded using an actophotometer that is square or circular in shape. This equipment may be tested on both rats and mice [15].
Elevated zero maze
The elevated zero maze (EZM) is a sensitive behavioral test that may be used to identify antineophobic and anxiolytic effects of medications and to disclose an animal's neophobia or nervousness. This labyrinth is 40 cm high, black, and annular, with an inner diameter of 30 cm and an outside circumference of 45 cm. The mouse can explore a runway ring that is 6 cm wide and has four quadrants: two opposing "open" quadrants with no walls and two opposing "closed" quadrants with walls that are 12 cm high. To keep the mouse from falling off, the open quadrants contain ridges of two to three millimeters. The thickness of the walls is 0.75 cm. For six minutes, each animal was put in a closed arm with its back to the open arm, and the following parameters were recorded [16].
Mirrored chamber
A quick, easy, and quantifiable method for evaluating anti-anxiety medications is the mirrored chamber test. The device consists of a square wooden box with a mirror of a cube that is open on one side. The thirty-cm-long mirrored cube is made up of five pieces of mirrored glass, one of which is mirrored and the other is dark brown. The inner cube was facing the floor pane, the top pane, and the three mirrored sides. 40 cm by 40 cm by 30 cm is the size of the container box. In order to create a 5 centimeter corridor that entirely encloses the mirrored chamber, the mirrored cube is positioned in the middle of the wooden container. Additionally, a mirror is positioned on the container sides facing the mirrored chamber's only open side [17].
Biochemical estimation
Collection of blood samples
On 14th d, blood (0.3 ml) was withdrawn from tail vein from all groups of mice. Blood samples were centrifuged at 2500 rpm for 10 min using refrigerated centrifuge (Paramount scientific works, Ambala cantt, India) to separate the plasma, which was used for estimation of corticosterone levels.
(a) Estimation of plasma corticosterone levels
The amount of corticosterone in the blood plasma was quantitatively estimated. After immersing the tubes in cold water for five minutes, 0.50 ml of 0.10 N sodium hydroxide was added to 1.0 ml of the sample in ethanol along with 0.50 ml of a 0.10 percent solution of p-nitroso-N,N-dimethylaniline in ethanol. After being sealed with cotton-wool, the tubes were left in a dark place at 0 °C for five hours. 2.0 ml of pH 9.8 buffer, 5.0 ml of a 0.10 % phenol in ethanol solution, and 0.50 ml of a 1.0 % potassium ferricyanide aqueous solution were added to the aforesaid solution. For ten minutes, the tubes were submerged in a water bath at 20±2 °C. A UV-visible spectrophotometer (UV 3200 UV-VIS Spectrophotometer, Somajiguda, Hyderabad) was used to read the solution at 650 nm.
Statistical analysis
Mean±SEM was used to express all of the data. Graph pad prism in stat (Graph Pad Software Inc., USA) was used to analyze the data for each group using a one-way ANOVA and Turkey's test. A significance level of p<0.05 was applied.
RESULTS
Effect of Rubia cordifolia extract on body weight (g) of mice
The mice of Rubia cordifolia extract (300 and 600 mg/kg/p. o.) treated group showed significantly (p<0.05) increased in body weight as compared to the control group. Treatment with APZ (0.25 mg/kg i. p) the body weight significantly increased as compared to normal group.
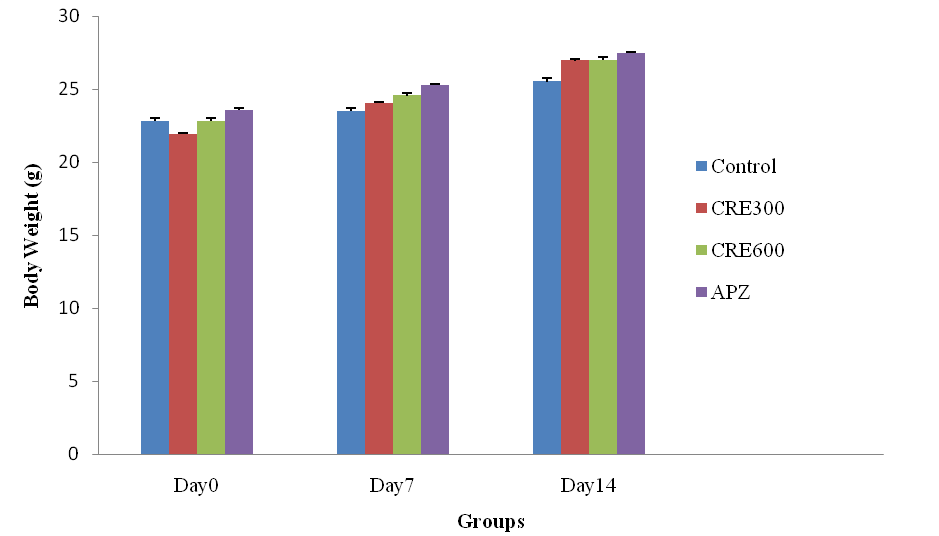
Fig. 1: Effect of Rubia cordifolia extract on body weight (g) of mice, values are expressed as mean±SEM adenotes p<0.05 as compared with to normal control group and bDenotes p<0.05 as compared to APZ (0.25 mg/kg i. p) treated group (One way ANOVA followed by Tukey’s test)
Effect of Rubia cordifolia extract on feed intake (g) of mice
The mice of Rubia cordifolia extract (300 and 600 mg/kg/p. o.) treated group showed significantly (p<0.05) increased in feed intake as compared to the control group. Treatment with APZ (0.25 mg/kg i. p) the feed intake significantly (p<0.05) increased as compared to control group group.
Effect of Rubia cordifolia extract on water intake (ml) of mice
The mice of Rubia cordifolia extract (300 and600 mg/kg/p. o.) treated group showed significantly (p<0.05) increased in body water intake as compared to the control group. Treatment with APZ (0.25 mg/kg i. p) the water intake significantly (p<0.05) increased as compared to caffeine group.
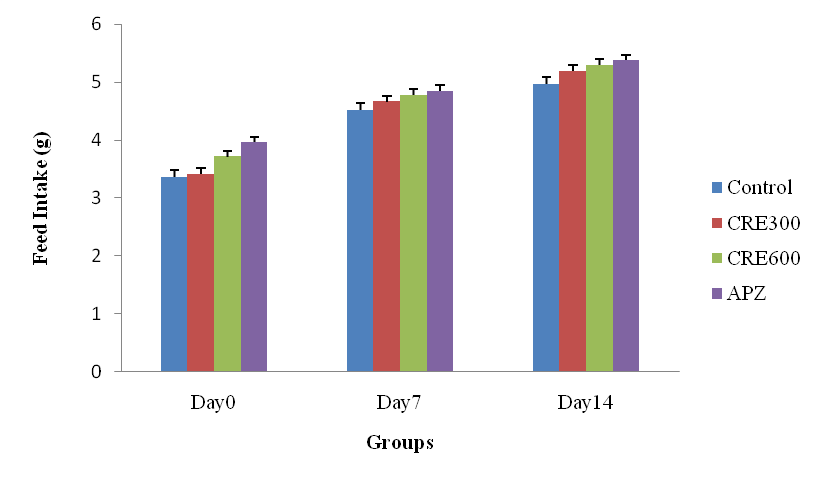
Fig. 2: Effect of Rubia cordifolia extract on feed intake (g) of mice, values are expressed as mean±SEM adenotesp<0.05 as compared with to normal control group and bdenotes p<0.05 as compared to APZ (0.25 mg/kg i. p) treated group (One way ANOVA followed by Tukey’s test)
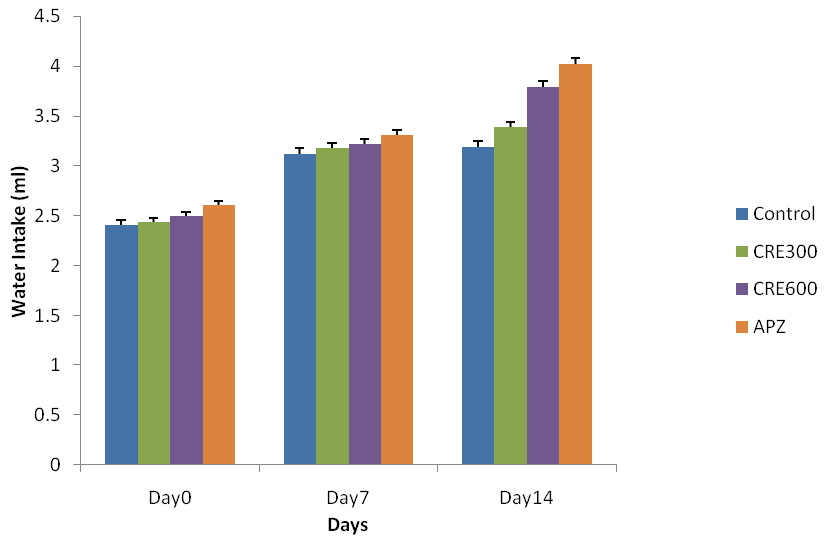
Fig. 3: Effect of Rubia cordifolia extract on water intake (ml) of mice, values are expressed as mean±SEM adenotes p<0.05 as compared with to normal control group and bdenotes p<0.05 as compared to APZ (0.25 mg/kg i. p) treated group (One way ANOVA followed by Tukey’s test)
Effect of Rubia cordifolia extract on locomotors activity of mice
The mice of Rubia cordifolia extract (300 and 600 mg/kg/p. o.) treated group showed no significantly (p<0.05) difference in locomotors activity as compared to control group. Treatment with APZ (0.25 mg/kg i. p) also showed no significant change as compared to control group.
Elevated zero maze
Effect of Rubia cordifolia extract on latency to enter in open arm (LEO)
An decrease in LEO than control indicates induction of anxiety and vice-versa. The mice of Rubia cordifolia extract (300 and 600 mg/kg/p. o.) treated group showed decreased (p<0.05) LEO as compared to normal control group. However pretreatment with APZ (0.25 mg/kg i. p) for 14 d also reduced (p<0.05) the LEO.
Effect of Rubia cordifolia extracts number of entries in open arm (NEO)
NEO indicates how many times the mouse entered in the open arm. Higher the frequency of entry in open arm, lower the anxiety level and vice versa. The mice of Rubia cordifolia extract ((300 and 600 mg/kg/p. o.) treated group showed increased (p<0.05) NEO as compared to normal control group. However pretreatment with APZ (0.25 mg/kg i. p) 14 d also increased the NEO.
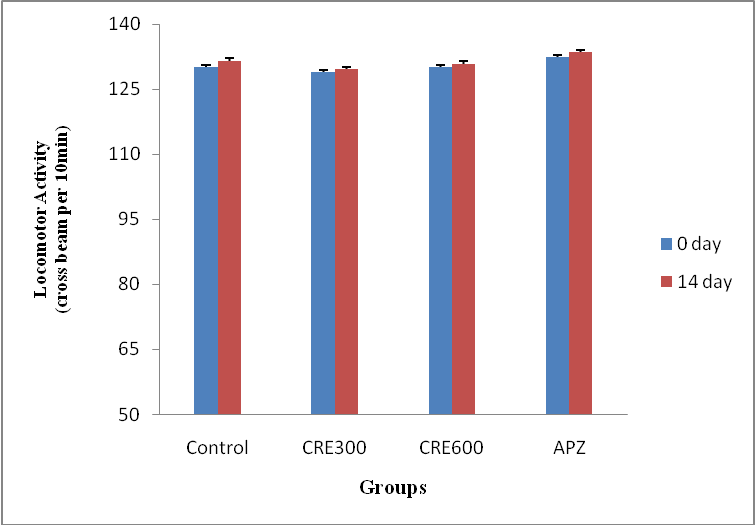
Fig. 4: Effect of Rubia cordifolia extract on locomotor activity of mice, values are expressed as mean±SEM (One way ANOVA followed by Tukey’s test)
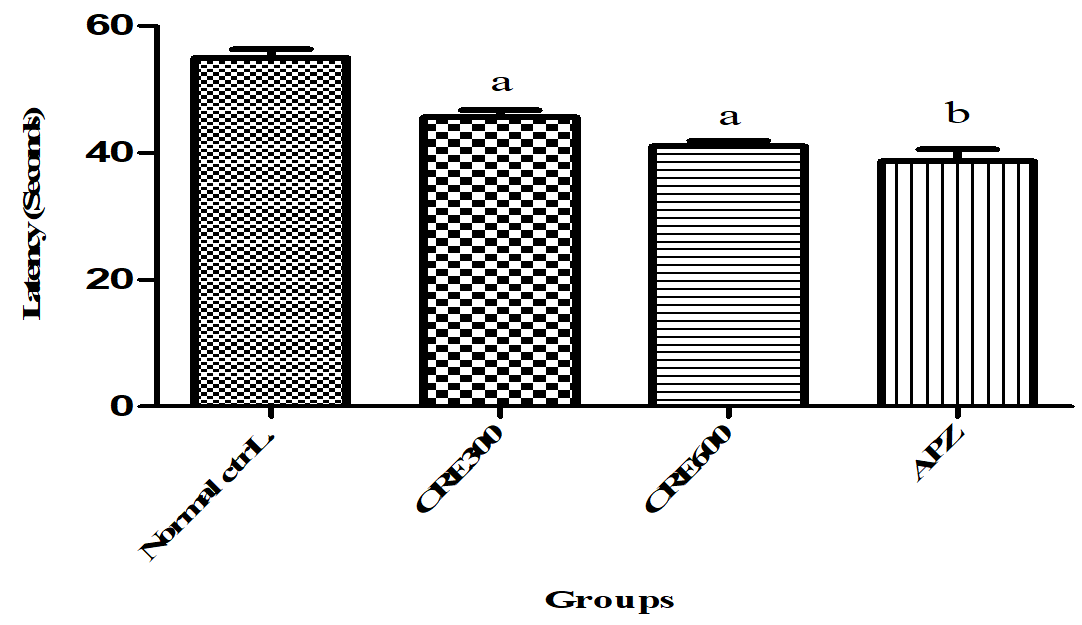
Fig. 5: Effect of Rubia cordifolia extract on LEO in elevated zero maze test, values are expressed as mean±SEM adenotesp<0.05 as compared with to normal control group and b denotes p<0.05 as compared to APZ (0.25 mg/kg i. p) treated group (One way ANOVA followed by Tukey’s test)
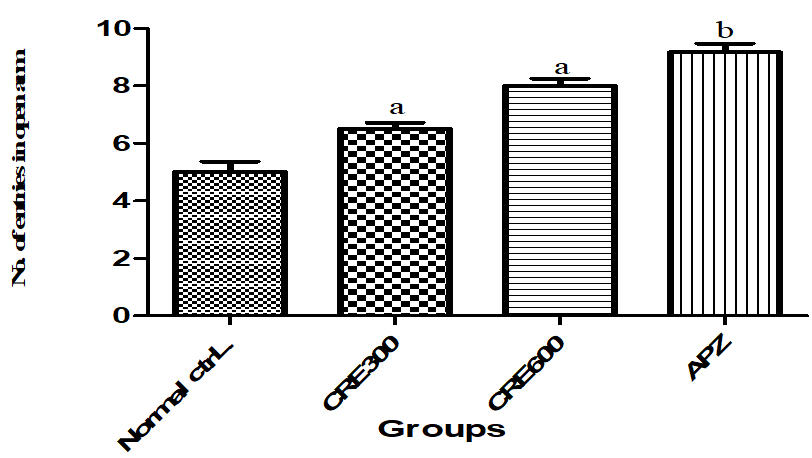
Fig. 6: Effect of Rubia cordifolia extract on NEO in elevated zero maze test, values are expressed as mean±SEM adenotes p<0.05 as compared with to normal control group and bdenotes p<0.05 as compared to APZ (0.25 mg/kg i. p) treated group (One way ANOVA followed by Tukey’s test)
Effect of Rubia cordifolia extract on time spent open arm (TSO)
TSO indicates the average time spent in open arm by the animal out of total 360 seconds. More the time spent by animal in open arm, lower the anxiety and vice-versa. The mice of Rubia cordifolia extract (300 and 600 mg/kg/p. o.) treated group showed increased (p<0.05) in TSO as compared to normal control group. However pretreatment with APZ (0.25 mg/kg i. p) 14 d increased the TSO significantly.
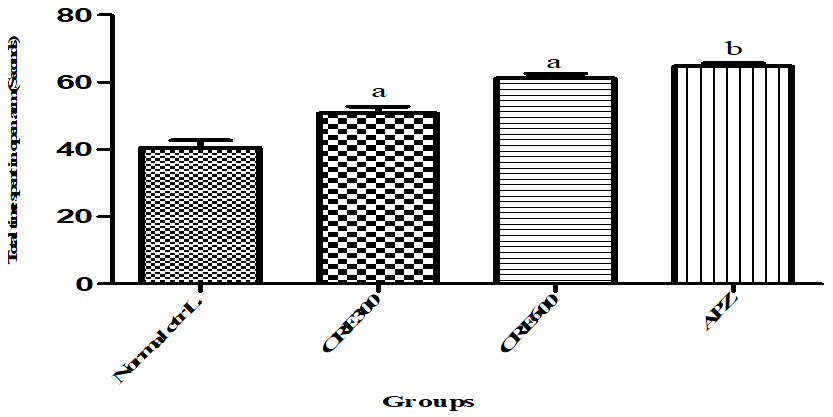
Fig. 7: Effect of Rubia cordifolia extract on TSO in elevated zero maze test, values are expressed as mean±SEM adenotes p<0.05 as compared with to normal control group and bdenotes p<0.05 as compared to APZ (0.25 mg/kg i. p) treated group (One way ANOVA followed by Tukey’s test)
Mirrored chamber
Effect of Rubia cordifolia extract on latency to enter in mirrored chamber
LEMC is the time taken by animal for first entry into the mirrored chamber. An increase in LEMC than control indicates induction of anxiety and vice-versa. The mice of Rubia cordifolia extract (300 and 600 mg/kg/p. o.) treated group showed decreased (p<0.05) LEMC as compared to normal control group. Pretreatment with APZ (0.25 mg/kg i. p) 14 d reduced (p<0.05) the LEMC significantly.
Effect of Rubia cordifolia extract on number of entries in mirrored chamber (NEMC)
NEMC indicates how many times the mouse entered in the mirrored chamber. Higher the frequency of entry in mirrored chamber, lower the anxiety level and vice versa. The mice of Rubia cordifolia extract (300 and 600 mg/kg/p. o.) treated group showed increased (p<0.05) NEMC as compared to normal control group. Pretreatment with APZ (0.25 mg/kg i. p) 21 d show a increased (p<0.05) the NEMC significantly.
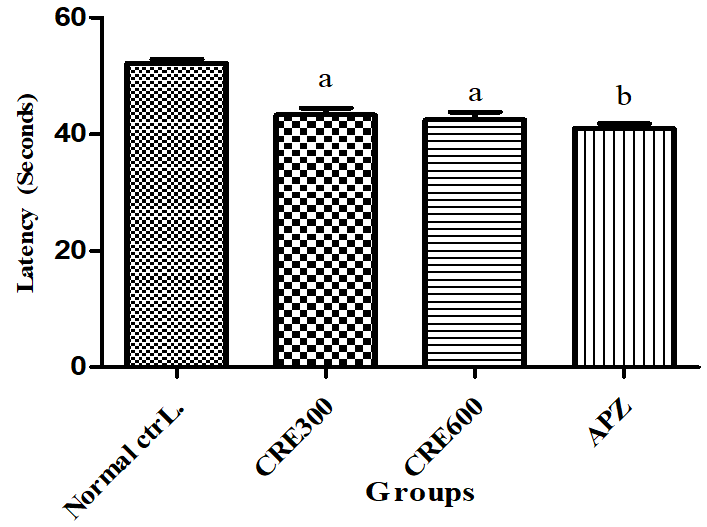
Fig. 8: Effect of Rubia cordifolia extract on LEMC, values are expressed as mean±SEM aDenotes p<0.05 as compared with to normal control group and bDenotes p<0.05 as compared to APZ (0.25 mg/kg i. p) treated group (One way ANOVA followed by Tukey’s test)
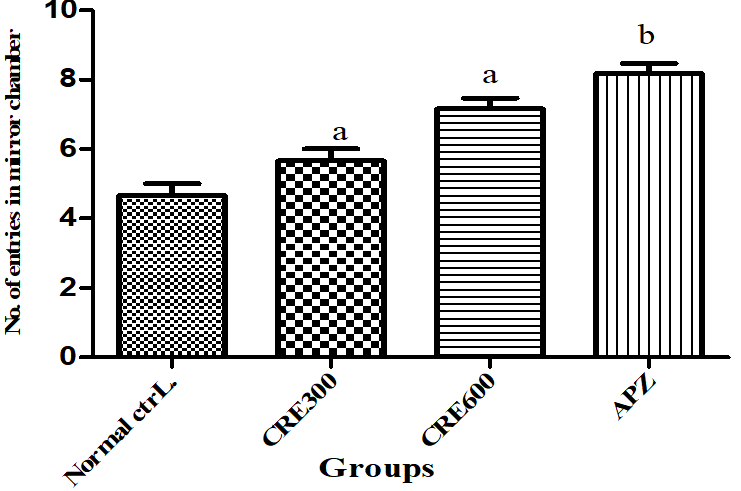
Fig. 9: Effect of Rubia cordifolia extract on NEMC, values are expressed as mean±SEM aDenotes p<0.05 as compared with to normal control group and bDenotes p<0.05 as compared to APZ (0.25 mg/kg i. p) treated group (One way ANOVA followed by Tukey’s test)
Effect of Rubia cordifolia extract on time spent in mirrored chamber (TSMC)
TSMC indicates average time spent in mirrored chamber by the animal out of total 300 seconds. More the time spent by animal in mirrored chamber, lower the anxiety and vice-versa. The mice of Rubia cordifolia extract (300 and 600 mg/kg/p. o.) treated group showed increased (p<0.05) in TSMC as compared to normal control group. Pretreatment with APZ (0.25 mg/kg i. p) 14 d increased (p<0.05) the TSMC significantly.
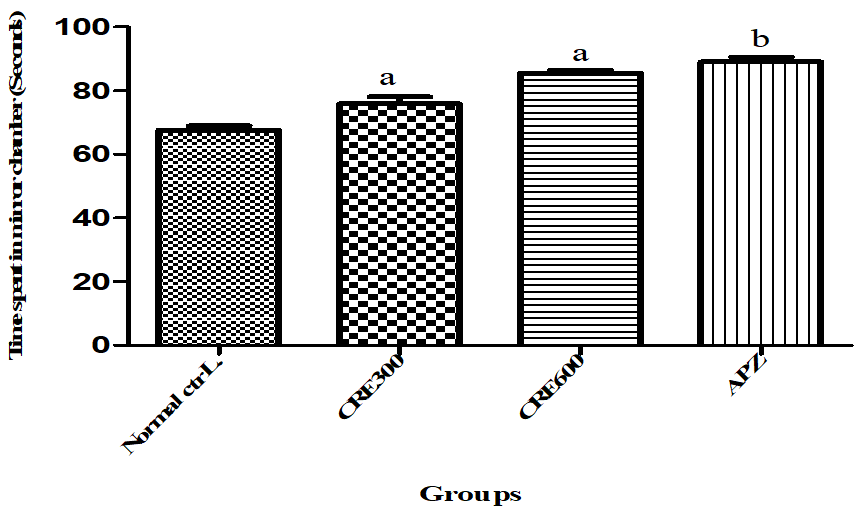
Fig. 10: Effect of Rubia cordifolia extract TSMC, values are expressed as mean±SEM aDenotes p<0.05 as compared with to normal control group and bDenotes p<0.05 as compared to APZ (0.25 mg/kg i. p) treated group (One way ANOVA followed by Tukey’s test)
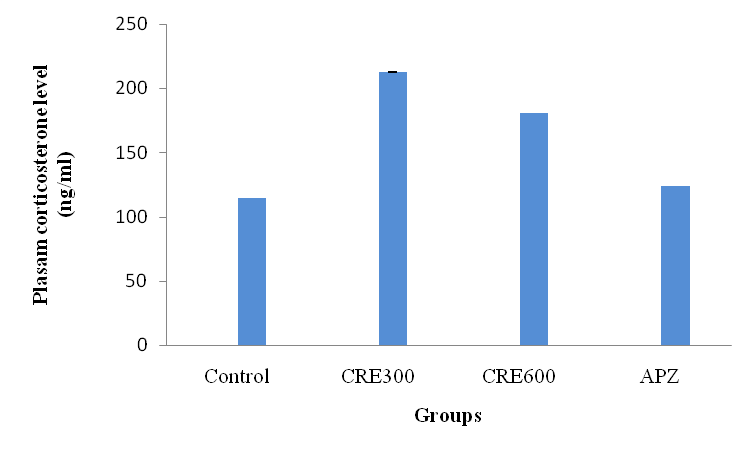
Fig. 11: Effect of Rubia cordifolia extract on plasma corticosterone levels, values are expressed as mean±SEM aDenotes p<0.05 as compared with to normal control group and bDenotes p<0.05 as compared to APZ (0.25 mg/kg i. p) treated group (One way ANOVA followed by Tukey’s test)
Effect of Rubia cordifolia extract on plasma corticosterone levels (CORT)
The mice of Rubia cordifolia extract (300 and 600 mg/kg/p. o.) treated group showed increased plasma corticosterone level (p<0.05) as compared to normal control mice. However pretreatment) APZ (0.25 mg/kg i. p) 14 d showed significant increased (p<0.05) in plasma corticosterone significantly.
CONCLUSION
The present “Study to investigate the anxiolytic effect of Rubia cordifolia Linn in mice” Male Swiss albino mice of age 6-8 w and weight 20-35 g were used in the present study. Rubia cordifolia extract will be administered p. o. to mice daily in three different doses (300 and 600 mg/kg/p. o.) for 14 d regularly. On 15th d, the animal subjected to locomotor activity, elevated zero maze, mirrored chamber test and plasma corticosterone level. Experimental animals as indicated by decrease LEO and increase in NEO as well as TSO in elevated zero maze studies. Also decrease LEMC and increased NEMC as well as TSMC in mirrored chamber studies. Administration of Rubia cordifolia extract will be administered p. o. to mice daily in three different doses extract (300and 600 mg/kg/p. o.) for 14 d significantly reduced anxiety. Thus, Rubia cordifolia extract may prove to be useful remedy for the management of anxiety owing to its possible neuroprotective and its antioxidant properties.
ACKNOWLEDGMENT
It’s our privilege to express the profound sense of gratitude and cordial thanks to our respected chairman Mr. Anil Chopra and Vice Chairperson Ms. Sangeeta Chopra, St. Soldier Educational Society, Jalandhar for providing the necessary facilities to complete this research work.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
All the authors have contributed equally
CONFLICT OF INTERESTS
Declared none
REFERENCES
Bleakley S, Maguire T. Anxiety disorders: clinical features and diagnosis. Pharm J. 2013;5:281.
Soodan S, Arya A. Understanding the pathophysiology and management of the anxiety disorders. Hum J Rev Artic. 2015;4:251-78.
Sahib S, Alexander B, Michale E, Jason S. Current diagnosis and treatment of anxiety disorders. PT. 2013;38(1):30-57. PMID 23599668.
Neil A, Kitchen K, Danielle B. Anxiety disorder: an information guide. Vol. 39. Toronto: Centre for Addiction and Mental Health; 2011. p. 1-37.
Lepine JP. The epidemiology of anxiety disorders: prevalence and societal costs. J Clin Psychiatry. 2002;63 Suppl 14:4-8. PMID 12562112.
Cannistraro PA, Rauch SL. Neural circuitry of anxiety: evidence from structural and functional neuroimaging studies. Psychopharmacol Bull. 2003;37(4):8-25. PMID 15131515.
Arikian SR, Gorman JM. A review of the diagnosis pharmacologic treatment and economic aspects of anxiety disorders. Prim Care Companion J Clin Psychiatry. 2001;3(3):110-7. doi: 10.4088/pcc.v03n0302, PMID 15014608.
Justin M, Sanjay J, Jack M. Molecular targets in treatment of anxiety. Biol Psychiatry. 2002 Nov 15;52(10):1008-38. doi: 10.1016/S0006-3223(02)01672-4.
Zeind CS, Carvalho MG, Cheng JW, Zaiken K, LaPointe T. Applied therapeutics: the clinical use of drugs. Lippincott Williams & Wilkins; 2023 Jan 6.
Wang HR, Woo YS, Bahk WM. Caffeine induced psychiatric manifestations: a review. Int Clin Psychopharmacol. 2015 Jul 1;30(4):179-82. doi: 10.1097/YIC.0000000000000076, PMID 25856116.
Indian Materia Medica. K. M. Nadkarni. Vol. 1. Bombay: Popular Prakashan; 2007. p. 1‑37.
Kirtikar KR, Basu BD. Indian medicinal plants. 2nd ed. Bhuwaneswari Asrama; 1918.
Arikian SR, Gorman JM. A review of the diagnosis pharmacologic treatment and economic aspects of anxiety disorders. Prim Care Companion J Clin Psychiatry. 2001;3(3):110-7. doi: 10.4088/pcc.v03n0302, PMID 15014608.
Chitra V, Pavan Kumar K. Neuroprotective studies of Rubia cordifolia Linn. On β-amyloid induced cognitive dysfunction in mice. Int J Pharm Tech Res. 2009 Oct;1(4):1000-9.
Kulkarni SK, Dandiya PC. Influence of intraventricular administration of norepinephrine dopamine and 5-hydroxytryptamine on motor activity of rats. Indian J Med Res. 1975;63(3):462-8. PMID 1213741.
Shepherd JK, Grewal SS, Fletcher A, Bill DJ, Dourish CT. Behavioural and pharmacological characterisation of the elevated “zero-maze” as an animal model of anxiety. Psychopharmacology. 1994 Sep;116(1):56-64. doi: 10.1007/BF02244871, PMID 7862931.
Reddy DS, Kulkarni SK. Differential anxiolytic effects of neurosteroids in the mirrored chamber behavior test in mice. Brain Res. 1997;752(1-2):61-71. doi: 10.1016/s0006-8993(96)01447-3, PMID 9106441.