
Int J Curr Pharm Res, Vol 17, Issue 6, 52-56Original Article
EVALUATION OF SERUM PROLACTIN LEVEL IN CIRRHOSIS OF LIVER AND ITS CORRELATION WITH CHILD-TURCOTTE-PUGH SCORE
ADITYA GOYAL1*, PANKAJ BANSAL2, ABHISHEK DEEPAK3
1,2Department of General Medicine, SMSR, Sharda University, Greater Noida, Uttar Pradesh, India. 3Department of General Medicine, SMSR, Sharda University Greater Noida, Uttar Pradesh, India
*Corresponding author: Aditya Goyal; *Email: adityagoyal97@gmail.com
Received: 15 Aug 2025, Revised and Accepted: 08 Oct 2025
ABSTRACT
Objective: The objective of this study was to determine serum prolactin levels in patients with liver cirrhosis and evaluate its correlation with the Child-Turcotte-Pugh (CTP) score.
Methods: This observational cross-sectional study was conducted at the Department of General Medicine, School of Medical Sciences and Research, Sharda University, Greater Noida. A total of 50 cirrhotic patients fulfilling the inclusion criteria were enrolled. Each participant provided informed consent, followed by a detailed clinical assessment and biochemical tests.
Results: The majority of patients fell within the 41–50 age group. Alcohol was identified as the most prevalent cause of cirrhosis, followed by Hepatitis B virus (HBV), Hepatitis C virus (HCV), and Non-Alcoholic Steatohepatitis (NASH). The mean prolactin levels observed for Child-Pugh grades A, B, and C were 25±2.83, 44.30±9.52, and 76.35±33.84 respectively. A statistically significant correlation (p<0.05) was found between prolactin levels and the Child-Pugh score (r=0.42, p=0.002), indicating that higher disease severity corresponded with elevated prolactin levels.
Conclusion: Serum prolactin levels demonstrated a strong association with the Child-Pugh score, signifying their potential as an early marker for liver cirrhosis severity and prognosis.
Keywords: Serum, Cirrhosis of liver, Its correlation
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijcpr.2025v17i6.7076 Journal homepage: https://innovareacademics.in/journals/index.php/ijcpr
INTRODUCTION
Nearly 35 million people worldwide pass away from chronic illnesses, which account for roughly 46% of all diseases and 59% of all deaths, according to the WHO. Over time, the prevalence of liver disease has been rising steadily. Studies on the prevalence of cirrhosis in the general population show that it ranges from 4.5% to 9.5% worldwide [1].
In addition to portal hypertension (P-HTN), spontaneous bacterial peritonitis, porto-systemic shunt, ascites, bleeding from varices, hemorrhagic syndrome, hepatic encephalopathy, and hepatocellular carcinoma (HCC), liver cirrhosis can also cause splenomegaly, cholestasis, and jaundice [2]. An abrupt loss of weight, fever, nausea, weakness, and loss of appetite are all early symptoms of cirrhosis. As liver function deteriorates, other well-known cirrhosis symptoms like simple bleeding and bruising start to show up. These include jaundice, rough skin, and edema (swelling) in the ankles, feet, and legs. Ascites, altered feces and urine, disorientation, fogging of the brain, memory loss, and changes in personality, Spider-like blood vessels surrounding a small, red skin lesion, Male symptoms include shrunken testicles, gynecomastia, and loss of sex desire, while women experience early menopause [3].
Patients with cirrhosis often experience significant adverse effects that could be fatal. The disease is also associated with a lower quality of life, a reduced capacity to work, easy fatigability, a higher risk of mild hepatic encephalopathy, sexual dysfunction, and trouble sleeping [4]. Liver cirrhosis is linked to a number of endocrine system abnormalities, primarily believed to be brought on by the diseased liver's inefficient hormone removal. It is now understood that the pathophysiology of liver cirrhosis's disrupted hormone activity is more intricate and includes modified secretion and feedback pathways [5].
Because liver transplantation is the only sure and preferred treatment for liver cirrhosis, and because it is difficult to perform due to donor shortages and high costs, finding a marker associated with disease severity benefits in clinical care [6]. Numerous non-invasive biochemical examinations, such as the FORNS index, fibrospect, and the aspartate aminotransferase and alanine aminotransferase ratio (AAR), necessitate intricate computations and biochemical testing [7, 8]. A straightforward, easily accessible, repeatable, affordable, and precise non-invasive diagnostic tool for hepatic fibrosis is ideal [9]. Prolactin is one such hormone in this regard. Human prolactin is a pituitary hormone whose biological effects are solely related to lactation and reproductive processes, and whose production (i. e., serum levels) is regulated by dopamine [10]. There has been much discussion on the levels of prolactin in patients with hepatic impairment.
The decrease in dopamine levels in the tuber infundibular tract is the primary cause of the elevation of prolactin [11]. By directly affecting the anterior pituitary and interfering with the hypothalamus's dopamine secretion, estrogens promote the release of prolactin [12]. Therefore, one of the most important tools for early intervention in such circumstances is the use of a biomarker, like prolactin, whose levels give us an indication of the severity of the condition and the risk of consequences.
Child and Turcotte initially suggested the Child–Pugh score [13] as a way to estimate the operative risk for patients having portosystemic shunt surgery for variceal hemorrhage. Ascites, hepatic encephalopathy (HE), nutritional status, total bilirubin, and albumin were all included in the main Child-Pugh score. In clinical work, the Child-Pugh score has been frequently used to evaluate the degree of liver impairment.
Child-Pugh score is frequently used to forecast how cirrhotic patients may fare. They do have certain disadvantages, though. First, two of the Child-Pugh score's variables—ascites and HE—are arbitrary and could change based on the doctors' opinions as well as the usage of lactulose and diuretics. Second, coagulopathy and, by extension, liver function in liver cirrhosis are not adequately reflected by INR, one of the Child-Pugh scores. Third, the INR value varies between laboratories [14].
The purpose of this study was to determine whether serum prolactin is an early indicator of complications in patients with cirrhosis of the liver and to evaluate the relationship between serum prolactin levels and the severity of the disease (as determined by the Child Pugh system) in these patients.
MATERIALS AND METHODS
This observational cross-sectional was done on 50 patients above the age of 18 y from May 2023 to Nov 2024 with their full informed consent
Inclusion criteria
All patients aged more than 18 y of both genders.
Patients diagnosed with Cirrhosis of liver (on USG Liver is nodular or has irregular margins and splenomegaly).
Exclusion criteria
Age below 18 y.
Women with pregnancy.
Lactating mothers.
Patients with hepatocellular carcinoma.
Drugs causing hyperprolactinemia and hepatotoxicity.
Recent upper GI bleed.
Methodology
All participants were explained about the study.
All the patients meeting inclusion criteria underwent detailed assessment as following protocol and diagnosis of liver Cirrhosis was made on the basis of detailed history of the patients, complete systemic examination, including Cardiovascular, Respiratory, Central nervous system and Abdominal examination of the patients, were done with monitoring of vitals frequently and as the patient’s condition demands. All blood investigation was done, including renal function test, liver function test, complete blood count, HBsAg, HIV, HCV, PT/INR. Other investigations were urine analysis, ECG, chest X ray, USG whole abdomen (Liver is nodular/irregular margins and splenomegaly) and if required, CT abdomen. The patients were then scored based on the modified Child Pugh scoring system and divided into Classes A, B or C based on the score obtained.
Table 1: Child-Turcotte-Pugh score for cirrhosis of liver
| Measure | 1 point | 2 points | 3 points |
| Total bilirubin, µmol/l (mg/dl) | <34(<2) | 34–50(2–3) | >50(>3) |
| Serum albumin, g/dl | >3.5 | 2.8–3.5 | <2.8 |
| Prothrombin time, prolongation (s) | <4.0 | 4.0–6.0 | >6.0 |
| INR | <1.7 | 1.7–2.3 | >2.3 |
| Ascites | None | Mild (or suppressed with medication) | Moderate to severe (or refractory) |
| Hepatic encephalopathy | None | Grade I–II | Grade III–IV |
Table 2: Interpretation
| Points | Class | One-year survival | Two-year survival |
| 5–6 | A | 100% | 85% |
| 7–9 | B | 80% | 60% |
| 10–15 | C | 45% | 35% |
Statistical analysis
Data so collected was tabulated in an excel sheet, under the guidance of statistician. The means and standard deviations of the measurements per group were used for statistical analysis (SPSS 22.00 for windows; SPSS inc, Chicago, USA). For each assessment point, data were statistically analyzed using one-way ANOVA. The level of significance was set at p<0.05. Pearson correlation test was used to analyse correlation between the two variables.
RESULTS
Out of 50 subjects; male and female comprised of 82% and 18% of the subjects respectively. Hence there was male dominancy in the present study. Maximum subjects were from the age group of 41-50 y followed by 51-60 y (table 1).
In this study; most common cause of cirrhosis was alcohol followed by HBV, HCV along with NASH (Graph 1).
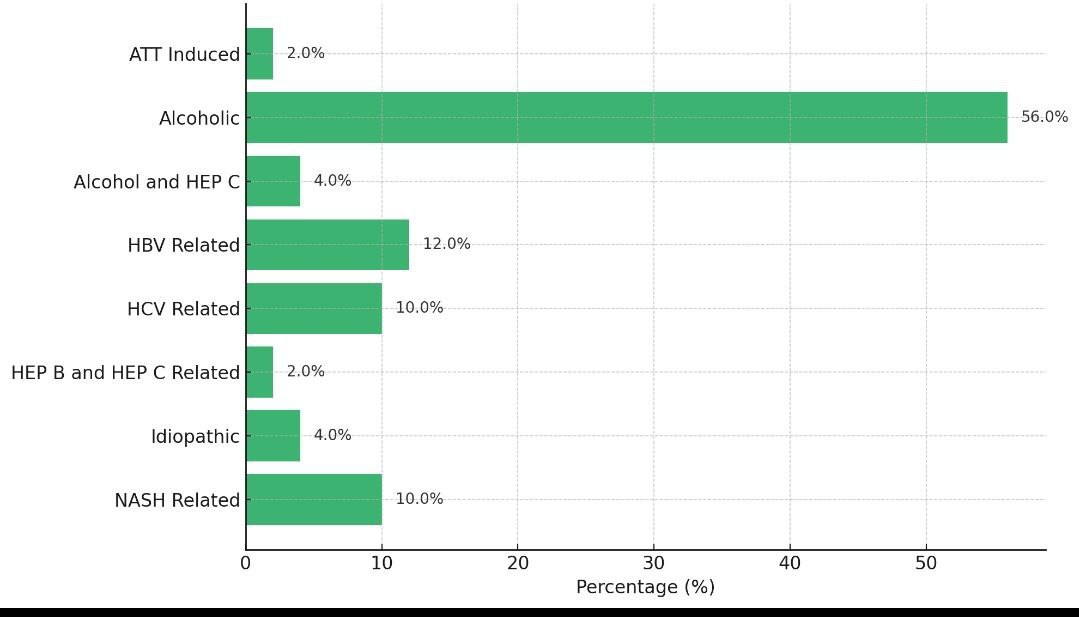
Graph 1: Cause of cirrhosis, mild, moderate and severe ascites was found in 28%, 24% and 46% of the subjects, respectively. Encephalopathy was present among 42% of the study subjects (table 4)
Table 3: Gender distribution among the study subjects
| Gender | N=50 | % |
| Male | 41 | 82 |
| Female | 9 | 18 |
| Age group (in years) | ||
| 19-30 | 5 | 10 |
| 31-40 | 8 | 16 |
| 41-50 | 17 | 34 |
| 51-60 | 14 | 28 |
| >60 | 6 | 12 |
Table 4: Ascites and encephalopathy distribution among the study subjects
| Ascites | N=50 | % |
| Absent | 1 | 2.0 |
| Mild | 14 | 28.0 |
| Moderate | 12 | 24.0 |
| Severe | 23 | 46.0 |
| Encephalopathy | ||
| No | 29 | 58 |
| Grade1 | 7 | 14 |
| Grade 2 | 9 | 18 |
| Grade 3 | 5 | 10 |
Mean HB, MCV, platelet (in k), serum albumin, total bilirubin, direct bilirubin and indirect bilirubin among the study subjects was 8.810±1.919, 100.536±7.9748, 85.32±51.1003, 2.6406±.659, 4.6678±5.725, 2.861±4.09 and 1.796±2.667 respectively, mean prolactin and Child Pugh score among the study subjects was 62.11±31.42 and 10.06±1.95, respectively (table 5).
Table 5: Descriptive analysis of investigative profile
| Variables | Minimum | Maximum | Mean | SD |
| Hb | 3.60 | 13.30 | 8.8100 | 1.91900 |
| MCV | 82.20 | 115.60 | 100.536 | 7.97480 |
| Platelet (in k) | 21.00 | 220.00 | 85.3200 | 51.10030 |
| Serum Albumin | 1.50 | 4.90 | 2.6406 | 0.65988 |
| Total Bilirubin | 0.18 | 22.00 | 4.6678 | 5.72515 |
| Direct Bilirubin | 0.11 | 17.62 | 2.8608 | 4.09059 |
| Indirect Bilirubin | 0.02 | 14.18 | 1.7960 | 2.66700 |
| PT | 15.00 | 54.00 | 24.9480 | 7.31488 |
| INR | 1.09 | 4.20 | 1.7348 | 0.53642 |
| Prolactin | 23.00 | 229.00 | 62.1140 | 31.41830 |
| Child Pugh Score | 6.00 | 14.00 | 10.0600 | 1.95260 |
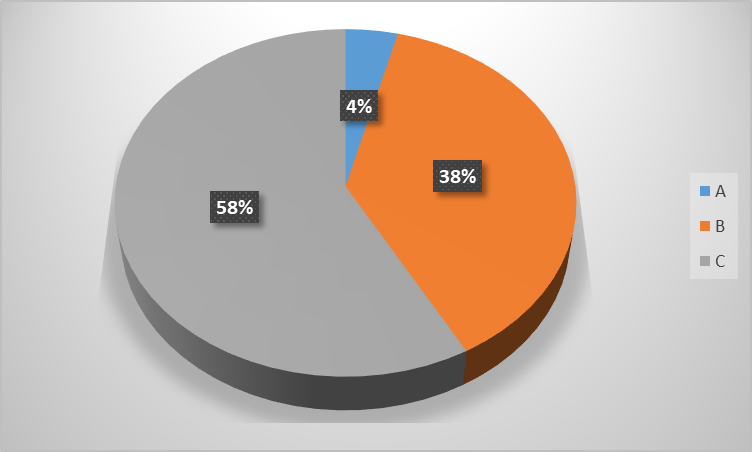
In our study, Child Pugh scores were classified into Class A (5-7), Class B (7-9) and Class C (10-15) respectively. Class A (5-7), Class B (7-9) and Class C (10-15) was reported among 4%, 38% and 58% of the subjects respectively (graph 2).
Graph 2: Child pugh grade among the study subjects
Mean prolactin value was 25±2.83, 44.30±9.52 and 76.35±33.84 in Child Pugh grade A, B and C, respectively. Hence there was increase in prolactin level with an increase in grade of Child Pugh score with statistically significant association as p<0.05 (table 4). It was found that significant positive correlation was found between prolactin level and Child Pugh score i. e. with increase in Child Pugh score, serum prolactin level also increases (r=0.42, p=0.002).
Table 6: Mean comparison of serum prolactin according to child pugh grade
| Child pugh grade | Mean prolactin | SD |
| A | 25.0 | 2.83 |
| B | 44.30 | 9.52 |
| C | 76.35 | 33.84 |
| p value | <0.01* |
*Statistically significant
DISCUSSION
Hepatic dysfunction and prolactin levels are always contentious. Dopamine was the primary neurotransmitter change that was reported. The fact that dopamine cannot be quantified in the brain or other bodily fluids limits its use. Few western studies have demonstrated prolactin as a predictive predictor since dopamine negatively regulates it 20. Between May 2023 and November 2024, 50 patients with liver cirrhosis who were enrolled in the OPD/IPD of General Medicine at Sharda University's School of Medical Sciences and Research in Greater Noida participated in the current cross-sectional study. Assessing serum prolactin levels in patients with liver cirrhosis and comparing them to the Child Pugh Turcotte score was the study's goal.
Out of 50 subjects; male and female comprised of 82% and 18% of the subjects, respectively. Males predominated in the current study as a result. Majority of the study's participants were between the ages of 41 and 50, then between the ages of 51 and 60. Prashant Punekar et al. [16] discovered that the largest percentage of participants (58.3%) were between the ages of 36 and 50. In their investigation, they also noted comparable male dominance. A comparable study conducted on 70 patients by Velissaris D et al. [11] revealed a male: female ratio of 2:1 and a median (range) age in years of 56 (34-68). In their study, Chaitanya H. Balakrishnan et al. [17] found that 60 patients had a male to female ratio of 5:1, with 75% of the patients being between the ages of 40 and 50. The results of studies by Khalil et al. [18] and Balakrishnan [17] are comparable to the present study.
According to this study, alcohol was the leading cause of CLD, followed by HBV, HCV, and NASH. In their study, Chaitanya H. Balakrishnan et al. [17] discovered that alcoholic liver disease was the most common cause of cirrhosis (73%), followed by hepatitis B infection (9%). Hepatitis B was the second most common cause of cirrhosis (55%) in a research by Prashant Punekar et al. [16]. Our study's results concurred with those of Balakrishnan [17].
However, hepatitis B virus was found to be the most common cause of liver cirrhosis (56%), according to a study by Hong et al. [19]. Of the individuals, 28% had mild ascites, 24% had moderate ascites, and 46% had severe ascites. Forty-two percent of the study participants had encephalopathy. Similar findings were made by Prashant Punekar et al. [16], who found that 47 (78.3%) of the cirrhosis patients had extensive ascites, 7 (11.7%) had moderate ascites, and 6 (10%) had no ascites. Nine cases (15%) and two cases (33%) had Grade II and Grade IV hepatic encephalopathy, respectively. Out of 26 trial participants, two, 14, and 8 patients, respectively, experienced ascites, splenomegaly, and esophageal varices, according to a study by Velissaris et al. [16].
The study participants' average Child Pugh score was 10.06±1.95. Child Pugh scores in our study were divided into three classes: Class A (5-7), Class B (7-9), and Class C (10-15). Of the subjects, 4% belonged to Class A (5-7), 38% to Class B (7-9), and 58% to Class C (10-15). According to a study by Chaitanya H. Balakrishnan et al. [17], 10% of the children in our study were in Class A, 40% were in Class B, and 50% were in Class C. 34.3% of patients with liver cirrhosis fell into group A of the Child Pugh classification, followed by B (22.9%) and C (42.9%), according to Velissaris D et al. [17]. In accordance with Prashant Punekar et al. [16], of the 60 patients, 36 (60%) developed cirrhosis and had a Child-Pugh score between 7 and 9, whereas 19 (31.7%) had a score of ≥10. Child-Pugh scores between 5 and 6 are found in just 5 (8.3%) of the patients. Twenty-two individuals were classified as Class A and four as Class B in the study by Velissaris et al. [11]. There were no patients in Class C of the Child-Pugh score. Based on the Modified Turcotte Child-Pugh score system, 10%, 40%, and 50% of the patients were classified as Class A, B, and C, respectively, according to Balakrishnan and Rajeev's findings [17].
The study participants' mean prolactin was 62.11±31.42. In Child Pugh grades A, B, and C, the mean prolactin values were 25±2.83, 44.30±9.52, and 76.35±33.84, respectively. As a result, there was a statistically significant correlation (p<0.05) between an increase in prolactin levels and an increase in Child Pugh score grade. Prolactin level and Child Pugh score were found to have a substantial positive association, meaning that as the Child Pugh score rose, so did the serum prolactin level (r=0.42, p=0.002). Human prolactin release typically exhibits a pulsatile pattern, but patients with liver cirrhosis have been seen to exhibit a continuous 24 h rise in prolactin release [20].
These results were comparable to those of a study by Arafa M et al. [21], who discovered that prolactin levels rose as the Child Pugh class went from A to C. According to Zeitz B et al. [22], patients with Child Pugh Class C had the highest levels of prolactin. In similar to our study, Chaitanya H. Balakrishnan et al. [17] found that 73.33% of patients had elevated serum prolactin levels, and that patients with higher Child Pugh classifications (B and C) also had higher prolactin levels.
In a similar vein, Prashant Punekar et al. [16] found a statistically significant correlation between prolactin levels and Child-Pugh scores (x2=12.2, P=0.003). Prolactin levels and CPS Grades B and C were shown to be strongly statistically significantly correlated (P=0.001) each. The results of Khalil et al. [18], who discovered a statistically significant relationship between blood prolactin levels and Child-Pugh grading 6, are comparable to our one. A research by Arafat et al. [21] found that when liver disorders progressed from Child-Pugh score A to C, the blood prolactin level increased significantly (P=0.023, P=0.000, and P=0.007, respectively). According to a study by Balakrishnan and Rajeev [17], patients in Class C had the highest serum prolactin levels.
LIMITATIONS
The existence of confounding variables, such as undiagnosed concomitant disorders, which may lead to high prolactin levels, is the main limitation of this study.
The limited sample size and study design are further disadvantages.
Comparisons between prolactin levels and other problems, such as hepatopulmonary syndrome and hepatorenal syndrome, require further investigation. Cohort studies can be used to investigate the relationship between high prolactin levels and mortality rates.
CONCLUSION
The study demonstrates that because serum prolactin levels closely match the Child Pugh scoring system, they can be used as a diagnostic for the severity of liver cirrhosis in patients. Therefore, we draw the conclusion that serum prolactin levels can serve as both an early warning sign of liver cirrhosis problems and a helpful prognostic marker in patients with the condition.
Studies that incorporate prolactin levels into prognostic scoring systems for liver disease must have a sizable sample size and follow-up. Prolactin levels and other liver disease consequences, like hepatopulmonary syndrome and hepatorenal syndrome, need to be compared in future research. To examine the relationship between high prolactin levels and death rates, cohort studies might be conducted.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
All authors have contributed equally
CONFLICT OF INTERESTS
Declared none
REFERENCES
Sarin SK, Maiwall R. Global burden of liver disease: a true burden on health sciences and economie. WGN Expert Point of View Articles Collection. 2012;17(2).
Song JY, Jung SJ, Park CW, Sohn JW, Kim WJ, Kim MJ. Prognostic significance of infection acquisition sites in spontaneous bacterial peritonitis: nosocomial versus community-acquired. J Korean Med Sci. 2006;21(4):666-71. doi: 10.3346/jkms.2006.21.4.666, PMID 16891810.
Cleveland Clinic. Cirrhosis of the liver: what it is symptoms causes and stages. Cleveland (OH): Cleveland Clinic. Available from: https://my.org/health/diseases/15572-cirrhosis-of-the-liver. [Last accessed on 19 Nov 2022].
Halkurike Jayadevappa VK, Goel A, Paliwal VK, Rai P, Aggarwal R. Liver disease severity is poorly related to the presence of restless leg syndrome in patients with cirrhosis. Neurol India. 2019;67(3):732-7. doi: 10.4103/0028-3886.263171, PMID 31347545.
Baker HW, Burger HG, De Kretser DM, Dulmanis A, Hudson B, O Connor S. A study of the endocrine manifestations of hepatic cirrhosis. Q J Med. 1976;45(177):145-78. doi: 10.1093/oxfordjournals.qjmed.a067451, PMID 769039.
Prakash BC, Shetty AS. Correlation between apri index meld score and child pugh score in cirrhosis of liver. Int J Adv Med. 2019;7(1):46-50. doi: 10.18203/2349-3933.ijam20195615.
Poynard T, Imbert Bismut F, Munteanu M, Messous D, Myers RP, Thabut D. Overview of the diagnostic value of biochemical markers of liver fibrosis (Fibro Test, HCV FibroSure) and necrosis in patients with chronic hepatitis C. Comp Hepatol. 2004;3(1):8. doi: 10.1186/1476-5926-3-8, PMID 15387887.
Fallatah HI. Noninvasive biomarkers of liver fibrosis: an overview. Adv Hepatol. 2014;2014:1-15. doi: 10.1155/2014/357287.
Lesmana CR, Salim S, Hasan I, Sulaiman AS, Gani RA, Pakasi LS. Diagnostic accuracy of transient elastography (FibroScan) versus the aspartate transaminase to platelet ratio index in assessing liver fibrosis in chronic hepatitis B: the role in primary care setting. J Clin Pathol. 2011;64(10):916-20. doi: 10.1136/jclinpath-2011-200044, PMID 21670074.
Bernichtein S, Touraine P, Goffin V. New concepts in prolactin biology. J Endocrinol. 2010;206(1):1-11. doi: 10.1677/JOE-10-0069, PMID 20371569.
Velissaris D, Karanikolas M, Kalogeropoulos A, Solomou E, Polychronopoulos P, Thomopoulos K. Pituitary hormone circadian rhythm alterations in cirrhosis patients with subclinical hepatic encephalopathy. World J Gastroenterol. 2008;14(26):4190-5. doi: 10.3748/wjg.14.4190, PMID 18636665.
Piercy M, Shin SH. Comparative studies of prolactin secretion in estradiol-primed and normal male rats induced by ether stress pimozide and TRH. Neuroendocrinology. 1980;31(4):270-5. doi: 10.1159/000123087, PMID 6775240.
Pugh RN, Murray Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60(8):646-9. doi: 10.1002/bjs.1800600817, PMID 4541913.
Srinivasa SV, Vemanamanda S. Correlation of relation of serum prolactin level to Child-Pugh score in cirrhosis of liver in assessing disease severity. J Med Chem Sci. 2024;7:34-41.
Mondal D, Das K, Chowdhury A. Epidemiology of liver diseases in India. Clin Liver Dis. 2022;19(3):114-7. doi: 10.1002/cld.1177, PMID 35355840.
Punekar P, Bhargava A, Ratre S, Choudhary S. Correlation of serum prolactin level to Child-Pugh scoring system and its prognostic significance in cirrhosis of liver. Asian J Med Sci. 2022;13(8):69-74. doi: 10.3126/ajms.v13i8.42953.
Balakrishnan CH, Rajeev H. Correlation of serum prolactin level to Child-Pugh scoring system in cirrhosis of liver. J Clin Diagn Res. 2017;11(7):OC30-3. doi: 10.7860/JCDR/2017/24730.10273, PMID 28892958.
Khalil FM, Elassal MA, Hussein AM, Rizk M, Awadein MA, Behiry EG. Serum prolactin level as a biological marker of severity in liver cirrhosis. Benha Med J. 2017;34(2):140. doi: 10.4103/bmfj.bmfj_60_17.
Hong WD, Dong LM, Jiang ZC, Zhu QH, Jin SQ. Prediction of large esophageal varices in cirrhotic patients using classification and regression tree analysis. Clinics (Sao Paulo). 2011;66(1):119-24. doi: 10.1590/s1807-59322011000100021, PMID 21437447.
Pravin Prabhu P. A study on correlation of serum prolactin level to child-pugh scoring system in cirrhosis of liver in assessing the severity of the disease. Doctoral Dissertation, Madurai Medical College, Madurai; 2018.
Arafa M, Besheer T, Elkannishy G, El hussiny MA, Rakha EB. Features of hormonal disturbances in cirrhotic patients with hepatic encephalopathy. Euroasian J Hepato Gastroenterol. 2012;2(2):84‑9.
Zeitz J, Mullhaupt B, Fruehauf H, Rogler G, Vavricka SR. Hepatic failure due to hepatitis B reactivation in a patient with ulcerative colitis treated with prednisone. Hepatology. 2009;50(2):653-4. doi: 10.1002/hep.23035, PMID 19575458.