
Int J Curr Pharm Res, Vol 17, Issue 6, 66-68Original Article
ROLE OF THERAPEUTIC PLASMA EXCHANGE IN YELLOW PHOSPHOROUS POISONING – A SINGLE CENTRE RETROSPECTIVE STUDY FROM SOUTH INDIA
VAIDESH G., PRATHIBA E. N., SHANTHINI GILDA V., RAVISHANKAR J.*
Department of Immunohematology and Blood Transfusion, Government Thoothukudi Medical College, Thoothukudi, Tamilnadu, India
*Corresponding author: Ravishankar J.; *Email: ravishankar@tvmc.ac.in
Received: 11 Aug 2025, Revised and Accepted: 06 Oct 2025
ABSTRACT
Objective: Yellow phosphorus, a rodenticide, is used as a suicidal agent that causes hepatotoxicity and acute liver failure (ALF) with increased mortality. The only definitive management is liver transplantation. Therapeutic plasma exchange (TPE) could alleviate the symptoms of yellow phosphorus by removing the poison and its metabolite from the body. Aim of this study was to determine the role and effectiveness of Therapeutic plasma exchange in yellow phosphorus poisoning.
Methods: This was a retrospective observational study conducted from January 2023 to December 2024. The study included patients who developed ALF due to yellow phosphorus poisoning requiring TPE. Patient demographic details, clinical features, quantity of consumption and laboratory values before and after TPE were noted. Statistical analysis was performed with Microsoft excel.
Results: 12 patients (M: F=2:1) who developed ALF, due to yellow phosphorus poisoning, were included for analysis. A total of 30 TPE sessions were performed (Mean=2.5 sessions). Mean age group of the patients was 29.3 years. Nine patients (75 %, M: F – 3.5:1) had recovery from ALF, out of which six had consumed<10 gm of yellow phosphorus. Among patients who recovered, mean time to admission was 2.8 days and mean time for initiation of TPE was 3.5 days. Three patients failed to show recovery and expired.
Conclusion: This study revealed that the patient outcomes were better with earlier initiation of TPE, but was also dependent on factors such as the quantity of poison consumed and time of hospitalization. Thus, TPE could potentially bridge the gap between medical management and liver transplantation in cases of yellow phosphorus poisoning.
Keywords: Acute liver failure, Therapeutic plasma exchange, Yellow phosphorus
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijcpr.2025v17i6.7083 Journal homepage: https://innovareacademics.in/journals/index.php/ijcpr
INTRODUCTION
Rodenticides, commonly referred to as “rat poisons,” are chemical agents aimed at eliminating small rodents like rats/mice, squirrels etc. Yellow Phosphorus poisoning is common in developing countries like India. It is often used as a suicidal agent or rarely by accidental ingestion [1, 2]. Once ingested, the clinical course is divided into three stages, which are somewhat similar to the of acetaminophen poisoning.
Stage 1 is the initial 24 h period following ingestion, where the patients have mild gastrointestinal symptoms, predominantly nausea or vomiting.
Stage 2 spans 24-72 h period, where the patients remain asymptomatic without any systemic manifestations.
Stage 3 is after the 72 h duration from the ingestion, where patients develop deranged liver functions, renal injury, coagulopathy progressing to ALF and hepatic encephalopathy (HE), shock and even death [3].
Yellow phosphorous (YP) is one of the rodenticides known to cause significant end‑organ damage and death. Fatal dose of Yellow Phosphorus ingestion is 1 mg/kg and fulminant poisoning occurs with doses exceeding 1–2 g/kg body weight. Acute Liver failure in rat killer paste poisoning (YP) patients requires liver transplantation as the treatment option if found unresponsive to medical management. Therapeutic plasma exchange (TPE) in this setting has the benefit to save a patient from a potential liver transplantation by removing the inflammatory mediators induced by the toxin/poison [2, 4].
Therapeutic plasma exchange (TPE) removes the circulating toxic waste products and replaces normal plasma at the same time, allowing us to manage the patients in ALF. TPE for Acute liver failure (ALF) is given category III, 2B indication as per American Society for Apheresis (ASFA) 2023 guidelines [5].
This study elaborates our experience in ALF patients due to Yellow Phosphorous (Ratol) poisoning, with a standard volume TPE as a modality when liver transplants are not easily available and to assess the effect of TPE on clinical parameters and patient outcome. The aim of the study was to determine the role and effectiveness of Therapeutic plasma exchange in cases of yellow phosphorus (Ratol) poisoning.
MATERIALS AND METHODS
This was a retrospective observational study conducted at the Department of Immunohematology and Blood Transfusion, Tirunelveli Medical College and Hospital, Tirunelveli. This study included data from patients who developed ALF, due to yellow phosphorus poisoning and underwent TPE between January 2023 to December 2024.
The initial evaluation of the patient was done by the treating physician at Intensive Medical Care Unit (IMCU) and the request for carrying out TPE was received at the Department of Transfusion Medicine. All patients who developed signs of acute liver failure following oral ingestion of yellow phosphorus poison and who gave informed consent for TPE. Patients who did not develop ALF after the yellow phosphorus poisoning and those did not complete TPE treatment were excluded from the study.
Written informed consent was obtained from all the patients for TPE and blood transfusion prior to TPE procedures. All the TPE sessions were carried out in the IMCU with continuous monitoring of patient blood pressure, heart rate, oxygen saturation, temperature, and general condition. The TPE sessions were performed by placing 11.5 fr central venous double‑lumen catheter in internal jugular vein or femoral vein. Acid citrate dextrose (ACD‑A) was the choice of anticoagulant used during the TPE procedure in all the patients. To minimize the adverse events related to ACD‑A anticoagulant, 10 ml of 10% calcium gluconate in normal saline was administered for all patients during the TPE procedure. 0.9% Normal saline was used as the replacement fluid during the procedure and the patients were transfused with an equal volume of Fresh Frozen Plasma (FFP) at the end of procedure.
The TPE procedures were carried out using Haemonetics MCS+LN 9000 Intermittent flow aphaeresis machine (Braintree, USA). One volume of plasma exchange was planned in all cases, but depending on the patient’s body weight, plasma volume, clinical condition and laboratory values, the amount of plasma volume exchanged varied from 1 to 1.5 l in this study. The endpoint for TPE was defined as resolution of coagulopathy, either normalization of liver enzyme levels or more than 75% reduction in liver enzyme levels, reduced requirement of vasopressors and no signs of hepatic encephalopathy.
Statistical analysis
Patient details like demographics, clinical features, quantity of consumption and laboratory values before and after TPE were collected from the patient case sheet at IMCU and therapeutic plasma exchange register at blood centre. Data were entered in Microsoft Excel and statistical analysis was done using SPSS version 22.0. Paired t‑test was used to compare the values pre and post-TPE TPE and P<0.05 was considered statistically significant.
RESULTS
The study included 12 patients (M: F = 2:1) who developed ALF due to yellow phosphorus poisoning. The mean age group of the patients was 29.3 y, with the highest prevalence among the age group of 20 to 30 y (fig. 1 and table 1). A total of 30 TPE sessions were performed (Mean=2.5 sessions).
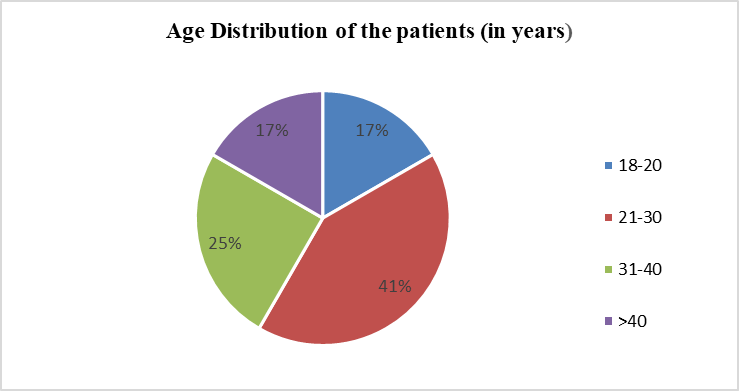
Fig. 1: Age distribution of patients with yellow phosphorus poisoning
Table 1: Age distribution of the study population
| Age (years) | Male | Female | Total |
| 18-20 | 0 | 2 | 2 |
| 21-30 | 4 | 1 | 5 |
| 31-40 | 3 | 0 | 3 |
| >40 | 1 | 1 | 2 |
| Total | 8 | 4 | 12 |
Table 2: Number of TPE cycles in patients
| No of TPE cycles | No of patients |
| 1 | 3 |
| 2 | 3 |
| 3 | 4 |
| 4 | 1 |
| 5 | 1 |
| Total | 12 |
Mean time interval between yellow phosphorus (ratol) consumption and day of admission was 2.8 days and initiation of TPE was 3.5 days. Nine patients (75 %, M: F = 3.5:1) had recovery from ALF, out of which six patients had consumed<10 gm of yellow phosphorus (ratol). Total bilirubin decreased (p = 0.002), SGOT decreased (p = 0.005), SGPT decreased (p = 0.007), PT declined (p = 0.004), APTT declined (p = 0.003) and INR also decreased (p = 0.006) post TPE (table 3). Three patients (M: F = 1:2) showed no improvement and expired during treatment (25%).
Table 3: Comparison of clinical parameters of the patients-pre and post TPE
| Clinical parameters | Pre TPE (mean) | Post TPE (mean) | p-value |
| T. Bilirubin (mg/dl) | 6.5 | 1.45 | 0.002 |
| SGOT (IU/l) | 285 | 59.3 | 0.005 |
| SGPT (IU/l) | 157 | 41.8 | 0.007 |
| PT (sec) | 26.7 | 15.5 | 0.004 |
| APTT (sec) | 36 | 27 | 0.003 |
| INR | 2.7 | 1.5 | 0.006 |
DISCUSSION
Rodenticides are classified based on their toxicity as highly toxic: Median lethal dose (LD 50) ranging from 0 to 50 mg/kg body weight, moderately toxic: LD 50 ranging from 50 to 500 mg/kg and less toxic: LD ≥ 500 mg/kg [1].
Yellow Phosphorus is a highly toxic rodenticide. Yellow phosphorus (Ratol) poisoning (YPP) is common in India as a suicidal agent and rarely by accidental ingestion. In the absence of an antidote for Yellow Phosphorus poisoning, the definitive treatment for acute liver failure is liver transplantation. But majority of patients cannot opt for a liver transplantation in the Indian setting due to various reasons like financial constraints, lack of organ donor registry and difficulty in finding a suitable organ donor in a very short span of time [6].
In ALF, TPE can remove albumin-bound toxins as well as unbound toxins, including aromatic amino acids, ammonia, endotoxin, indols, mercaptans, phenols, and other factors called damage-associated molecular patterns (DAMPs) responsible for organ failure, hepatic encephalopathy, and decreased systemic vascular resistance and cerebral blood flow. TPE has been used as adjunct or standalone therapy for bridging patients to recovery or LT [7].
In our study, YPP with ALF presentation was seen commonly in young individuals (between 20 and 40 y) with predominance in males. This was similar to the study by Nalabothu et al. [8]. The mean time to get admitted to the hospital was 2.8 days and mean time to start of plasma exchange was 3.5 days after YP consumption which were earlier when compared with study done by Angraje S et al. (3.6 and 4.86 days, respectively) [8]. The mean TPE sessions done were 2.5, which was similar to the studies done by Angraje S et al. (3.3 sessions) and Varghese J et al. (2.9 sessions) [8, 9].
There was a significant improvement in values of liver function tests and coagulation parameters between pre-and post-TPE values (p<0.05), which was similar to study done by Krishnamoorthy et al. [4]. The overall survival was 75 % which was similar to the studies done by Varghese J et al. (79% survival rate) and Angraje S et al. (70% survival rate) [8, 9].
Cheng et al. retrospectively studied 10 patients of Asian descent with ALF caused due to wide aetiologies treated with standard volume PE. Mean PE sessions were 5 with no significant improvement in coagulation parameters after PE with 30% survival [10].
Larsen et al., prospectively studied 92 patients of ALF, with a wide etiology, who were treated with TPE and showed a survival rate of 58.7% with a mean session of PE of 2.4. There was a statistically significant improvement in INR and enzyme levels post plasma exchange [11].
The study was a retrospective study and follow-up of the patients, who showed recovery following TPE, could not be documented. Further prospective randomized multi-center studies can help to validate these findings.
CONCLUSION
This study observed that TPE could potentially alleviate the symptoms caused by rat killer (YP) toxicity and bridge the gap between medical management and liver transplant. Patient outcomes depend on the amount of YP consumed and the time for initiation of TPE.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
All authors have contributed equally
CONFLICT OF INTERESTS
Declared none
REFERENCES
D Silva C, Krishna B. Rodenticide poisoning. Indian J Crit Care Med. 2019 Dec;23 Suppl 4:S272-7. doi: 10.5005/jp-journals-10071-23318, PMID 32021003.
Pahadia M, Saluja M, Bhushan B, Meena SR. Impact on morbidity and mortality by rodenticidal poisoning. Indian Med Gaz. 2013 May;147(5):170-4.
Ravikanth R, Sandeep S, Philip B. Acute yellow phosphorus poisoning causing fulminant hepatic failure with parenchymal hemorrhages and contained duodenal perforation. Indian J Crit Care Med. 2017 Apr;21(4):238-42. doi: 10.4103/ijccm.IJCCM_410_16, PMID 28515612.
Radhakrishnan K, Thokala RP, Anandan A, Rengan CS. Role of therapeutic plasma exchange in rat killer (yellow phosphorous) poisoning. Asian J Transfus Sci. 2023 Jan-Jun;17(1):74-8. doi: 10.4103/ajts.ajts_20_21, PMID 37188032.
Padmanabhan A, Connelly Smith L, Aqui N, Balogun RA, Klingel R, Meyer E. Guidelines on the use of therapeutic apheresis in clinical practice: evidence-based approach from the writing committee of the American Society for Apheresis: the eighth special issue. J Clin Apher. 2019 Jun;34(3):171-354. doi: 10.1002/jca.21705, PMID 31180581.
Eapen CE, Balasubramanian V, Ramamoorthy G, Jayanthi V, Sathiyasekaran M, Murugan N. Management of rodenticide poisoning: Tamil Nadu chapter of Indian society of gastroenterology guidelines. Gastroenterology Hepatology and Endoscopy Practice. 2022;2(1):1-6. doi: 10.4103/ghep.ghep_45_21.
Kandiah PA, Olson JC, Subramanian RM. Emerging strategies for the treatment of patients with acute hepatic failure. Curr Opin Crit Care. 2016 Apr;22(2):142-51. doi: 10.1097/MCC.0000000000000291, PMID 26849251.
Angraje S, Sekar M, Mishra B, Matcha J. Outcome of plasma exchange in acute liver failure due to yellow phosphorus poisoning: a single-center experience. Indian J Crit Care Med. 2021 Sep;25(9):1020-5. doi: 10.5005/jp-journals-10071-23971, PMID 34963720.
Varghese J, Joshi V, Bollipalli MK, Malleeswaran S, Patcha R, Nair H. Role of therapeutic plasma exchange in acute liver failure due to yellow phosphorus poisoning. Indian J Gastroenterol. 2020;39(6):544-9. doi: 10.1007/s12664-020-01095-y, PMID 33409946.
Cheng YL, Chang CH, Chen WT, Tsai MH, Lee WC, Tu KH. Prognostic factors and treatment effect of standard volume plasma exchange for acute and acute-on-chronic liver failure: a single-center retrospective study. Transfus Apher Sci. 2018 Aug;57(4):537-43. doi: 10.1016/j.transci.2018.05.030, PMID 29880246.
Larsen FS, Schmidt LE, Bernsmeier C, Rasmussen A, Isoniemi H, Patel VC. High-volume plasma exchange in patients with acute liver failure: an open randomised controlled trial. J Hepatol. 2016 Jan;64(1):69-78. doi: 10.1016/j.jhep.2015.08.018, PMID 26325537.