
Int J Pharm Pharm Sci, Vol 17, Issue 4, 1-11Original Article Article
ANALYTICAL METHOD DEVELOPMENT AND VALIDATION OF FLAVOXATE HCL WITH RP-HPLC
AAKRITI BHARDWAJ1, ROBIN KUMAR2, SHABNAM AIN1*, BABITA KUMAR1, QURRATUL AIN1
1Sanskar College of Pharmacy and Research, Ghaziabad-201001, Uttar Pradesh, India. 2Principle Scientific Officer, IPC, Ghaziabad-201001, Uttar Pradesh, India
*Corresponding author: Shabnam Ain; *Email: shabnam.ain@sanskar.org
Received: 16 Oct 2024, Revised and Accepted: 12 Feb 2025
ABSTRACT
Objective: To verify analytical methods, suitability for its intended use is the goal of validation by Reverse Phase-High Performance Liquid Chromatography (RP-HPLC) method.
Methods: A validated method for determining flavoxate hydrochloride was created using HPLC, which is a sensitive, exact, and straightforward technique. The inertsilC18 (150 mm×4.6 mm, 5µm) is used in this approach. The buffer, which included 3g of 1-hexane sulphonic acid, 3 ml of Orthophosphoric Acid (OPA) and 3 ml of Triethylamine (TMA), was combined with Acetonitrile (ACN) in a 650:350 ratio to form the mobile phase. At 293 nm, Ultraviolet (UV) detection was done.
Results: The Retaining time for flavoxate HCl was found to be 2.43 min. It was discovered that the linearity range of flavoxate HCl was 800-1200 µg/ml, and that the regression equation was y=17118x+80943. R 2 = 0.981 was found to be the linearity regression coefficient value. It was discovered that the RSD for intra-and inter-day precision was less than 2 %. Flavoxate HCl was discovered to have Limit of Detection (LOD)-83.13734 and Limit of Quantitation (LOQ)-251.9313 values of µg/ml and µg/ml, respectively. After statistical analysis, the results are deemed satisfactory.
Conclusion: This technique can be used to analyze bulk forms of the antimuscarinic medication flavoxate HCl with success.
Keywords: Accuracy, Flavoxate HCl, HPLC, Precision, Validation
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijpps.2025v17i4.53045 Journal homepage: https://innovareacademics.in/journals/index.php/ijpps
INTRODUCTION
Pharmaceutics is a subspecialty of pharmacy that studies how drugs are made into dosage forms. It is a methodical process to obtain a stable and effective formulation while taking into account all factors necessary to preserve its quality. The development, formulation, production, testing, and distribution of medication dosage forms are within the multidisciplinary field of pharmaceutics in pharmaceutical research. Physical pharmacy, pharmaceutical technology, biopharmaceutics, pharmacokinetics, and pharmaceutical analysis are only a few of the many topics it covers. For quality assurance and control throughout the medication research and production process, this field is essential [1-3].
Overactive Bladder (OAB) is a label for a group of urinary symptoms. The need to urinate might come on suddenly and be difficult to control. You may feel the need to urinate often during the day and night. Urgency incontinence refers to the unintentional loss of pee. Those who suffer from an OAB may experience feelings of insecurity. Fortunately, there is a treatment available, an artificial anticholinergic and antispasmodic drug called flavoxate HCl is used to treat OAB syndrome and urine problems. It is used to treat problems related to the bladder, including incontinence (leaking urine), painful urination, frequent or urgent urination, increased urination at night, and so on. Bladder infections, urethral irritation, enlargement of the prostate and OAB are the most common causes of these symptoms. Although flavoxate has a broad range of activity against muscarinic acetylcholine receptors, it is thought to have less ability to affect the central nervous system due to its highly polar quaternary ammonium group, which makes it less likely to pass lipid membranes like the Blood Brain Barrier (BBB) [4, 5].
Urine frequency and urgency are reduced and bladder capacity is increased by flavoxate. Since its approval for usage in 1970, flavoxate has been used in the US to treat OAB syndrome and cystitis symptoms [6, 7]. Flavoxate was once marketed under the brand name Uri spas and is available in 100 mg tablets in a number of generic variants. Parasympathetic stimulation frequently causes adverse symptoms such as dry mouth and eyes, headache, blurred vision, constipation, urine retention, impotence, tachycardia and palpitations, anxiety, restlessness, and in rare cases, agitation and delusions [8].
The ingredient "flavoxate hydrochloride" in flavoxate HCl relaxes the muscles in the bladder. As a result, it aids in the prevention of excessive or uncontrollably frequent urination. Drug use observation research included 1800 patients who received flavoxate (Spasuret®200) for urge incontinence over a two-week period. Parameters for effectiveness and tolerance were established. Flavoxate alone was used to treat a subset of 618 patients who did not have benign prostatic hyperplasia or urinary tract infections. Comparable to results from the full sample (1800 patients), the bladder capacity increased by 36% at the onset of urge sensation. The remaining urine volume was either steady or decreased in 89.2% of all individuals. In 1.8% of cases, undesirable side effects were experienced. When flavoxate was taken four times a day (800 mg) as opposed to three times a day (600 mg), both groups performed better. Flavoxate works effectively, is well tolerated, and has no negative side effects or leftover urine issues [9].
Modern pharmaceutical and biological analyses rely on High-Performance Liquid Chromatography (HPLC) as their primary separation method because to its very successful separations and generally high detection sensitivity. HPLC technique development and validation are critical to research, manufacturing, and drug discovery, among other applications [10-12]. Every facet of validation-related performance characteristic is covered in the International Conference on Harmonization (ICH) Guidelines for HPLC method validation. These include testing for system appropriateness, linearity, specificity, accuracy, precision, range and limit of finding, and limit of detection [13-15].
In the absence of established ways, methods are created for new products. A different approach to improve precision and durability for current (non-pharmacopoeia) products is to cut costs and turnaround times. When a different approach is suggested to replace the current protocol, comparative laboratory data with advantages and disadvantages are made accessible [16-18]. The primary active ingredient, any reaction impurities, all synthetic intermediates that are available, and any degradants are to be separated and quantified using the HPLC method as per validation of analytical procedures, text and methodology [19].
MATERIALS AND METHODS
Materials
A complimentary sample of flavoxate HCl was kindly supplied. The Indian Pharmacopeia's flavoxate HCl (2-Piperedin-1-ylethyl 3-methyl-4-oxo-2-phenylchromene-8-carboxylate) was used in Active Pharmaceutical Ingredient (API) for the investigative study. It's white. Flavoxate HCl is soluble in common organic solvents such as acetone, methanol, ethanol, chloroform, and ethyl ether but insoluble in water. It is within the class of antispasmodics, which are used to treat specific symptoms related to the bladder and urinary system.
Instrumentation
Experiments were conducted using a HPLC (Agilent 1260 Infinity) and a UV-Visible double-beam spectrophotometer (Perkin Elmer) an analytical balance (Metler Toledo) for weighing; we also used an ultrasonicator (Citizen Scales Pvt. Ltd.) to sonicate the standard and Product Sample Solutions.
Methods
Installation of RP-HPLC method
Choice of mobile phase
Many solvents, including methanol, chloroform, ethanol, acetone, hexane-sulphonic acid, ortho-phosphoric acid, triethylamine buffer, hexane-sulphonic acid, was evaluated for flavoxate HCl solubility. ACN along with buffer solution makes up the mobile phase. Together with their low cost and handy availability, the best solubility guided the choice of these two diluents as the mobile phase.
Preparation of mobile phase
Made a combination of Acetonitrile and Buffer in the ratio 65:35 v/v. Mixed well, sonicated by using Ultrasonicator (Citizen Scales Pvt. Ltd.) to degassed to get a clean Mobile Phase. Also, Mobile Phase is used as a Diluent and Blank.
Preparation of standard stock mixture
As a starting point, a standard stock solution of flavoxate HCl API was created with a concentration of 2000 ppm. Using a scale, 200 milligrams of the drug was taken to a 100-millilitre volumetric flask. After that, 15 milliliters of diluent were added to the volumetric flask, which was then shaken to ensure complete mixing before being placed in the sonicator for 10 min. Finally, the volume was made up with the diluent.
Preparation of standard solution
The standard solution consisted of a 1000 ppm solution of flavoxate HCl. In a 20-milliliter volumetric flask, 10 milliliters of the standard stock solution were added. Subsequently, a little quantity of diluent was introduced to it and subjected to brief sonication. Consuming the diluent, the volume was finally brought up to the mark.
Solution preparation for testing
From the 2000 ppm stock solution, 8 ml of an 800 PPM solution was placed into a 20 ml volumetric flask. After that, about 5 milliliters of diluent were enhanced to it and then sonicated for a duration of 10 min. Lastly, diluent was used to bring the volume to the specified level. A 9 ml solution with 900 PPM was transferred to a 20 ml volumetric flask from a 2000 ppm stock solution. After that, a diluent of around 5 ml was added to it and sonicated for a short duration (10 min). Utilizing the diluent, the volume was then brought to the required level.
A 20 ml volumetric flask was used to transfer 10 ml of a 1000 PPM solution from a 2000 ppm stock solution. I sonicated it for a long (10 min) after adding the minimal quantity of diluent, which was roughly 5 ml. Utilizing the diluent, the volume was then brought to the required level.
A 20 ml volumetric flask was used to transfer 11 ml of the 1100 PPM solution from the 2000 ppm stock solution. I sonicated it for a long (10 min) after adding the minimal quantity of diluent, which was roughly 5 ml. Utilizing the diluent, the volume was then brought to the required level.
From the 2000 ppm stock solution, 12 ml of the 1200 PPM solution was transferred to a 20 ml volumetric flask. I sonicated it for a long (10 min) after adding the minimal quantity of diluent, which was roughly 5 ml. utilizing the diluent, the amount was finally brought up to the sign.
Selection of detection of wavelength
The methanol was used to create the solution. We scanned the solution over the whole range (200-400) to get the UV spectra. Flavoxate HCl was chosen for detection because of its highest absorption at 293 nmλ Max.
Chromatographic conditions
In HPLC, chromatographic conditions are the particular parameters and configurations that are employed throughout the separation procedure. These circumstances have a major effect on the chromatographic analysis's overall success, resolution, and efficiency. The conditions such as system, software used, column etc. will show in table 1.
Table 1: Validation methods using optimised chromatographic conditions
| Chromatographic conditions | Validated method |
| HPLC System Used | Agilent’s 1200 Series |
| Software Used | EZ Chrome Elite |
| Column | Inertsil C18 (150 mm×4.6 mm,5µm) |
| Column Temperature | 30 °C |
| Flow Rate | 1.5 ml/min |
| Injection Level | 10µl |
| Wavelength | 293 nm |
| Run Time | 10 min |
| Mobile Phase | Buffer (1-hexane sulphonic acid, orthophosphoric acid, triethylamine), acetonitrile |
| Elution Mode | Isocratic |
RESULTS AND DISCUSSION
Verification of the flavoxate HCl optimised method using the RP-HPLC technique
For the RP-HPLC method of estimating flavoxate HCl, the C18 column was chosen. Optimization of the method was done. To achieve a good resolution, retention time, an adequate number of theoretical plates, and a tailing factor, the mobile phase ratio, flow rate, and pH were tuned. An isocratic combination of buffer, which contained 3g of 1-hexane sulphonic acid, 3 ml of orthophosphoric acid (OPA), and 3 ml of triethylamine (TMA), was combined with acetonitrile (ACN) in a 650:350 ratio to form the mobile phase. The flow rate chosen was 1.5 ml/min, and the retention time for flavoxate HCl was 2.43 min. This combination allowed the trial to achieve optimal separation. UV detection was done at 293 nm [20].
The developed HPLC technique forever olimus was validated by means of linearity and range, accuracy, specificity, limit of quantitation, repeatability, and intermediate precision and robustness, in accordance with ICHQ2 (R1) recommendations [20, 21].
System suitability
In HPLC, system appropriateness refers to a collection of standards used to make sure the HPLC system is operating properly and that the results are accurate and repeatable. Characteristics such as tailing factor, retention time, theoretical plates, and percentage Relative Standard Deviation (RSD) area in table 2.
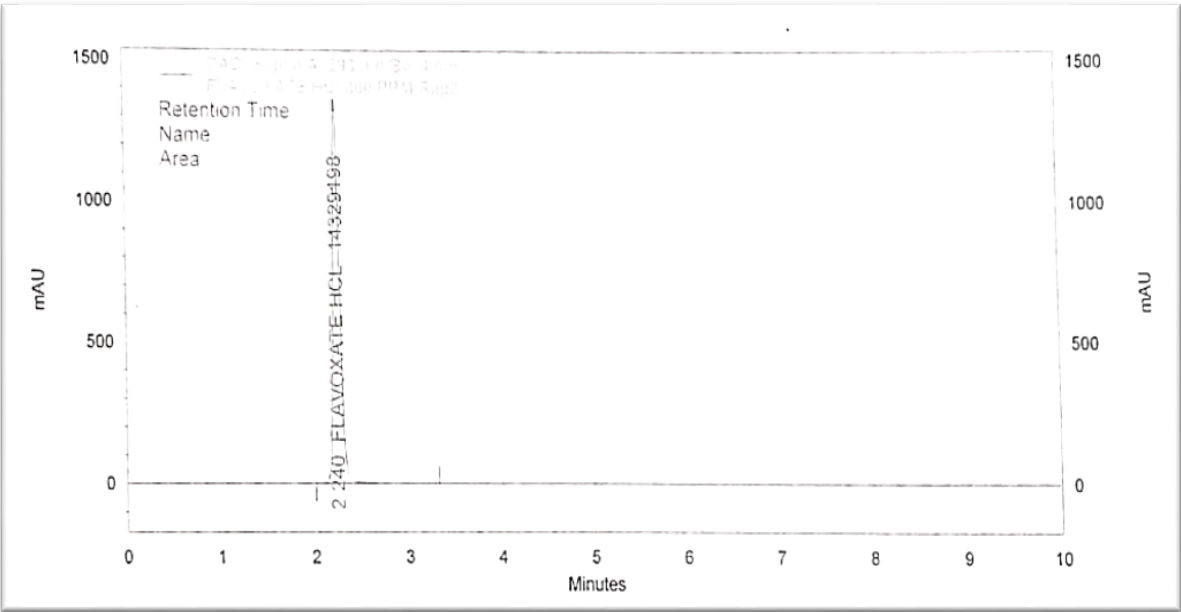
Fig. 1: Chromatogram of flavoxate HCl 800 PPM
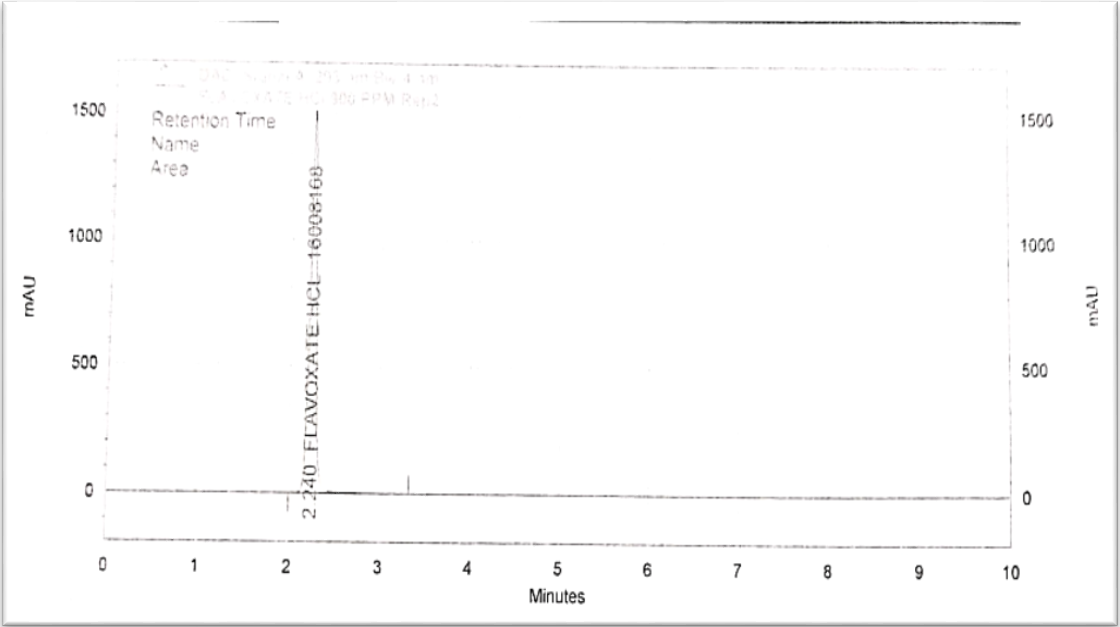
Fig. 2: Chromatogram of flavoxate HCl 900 PPM
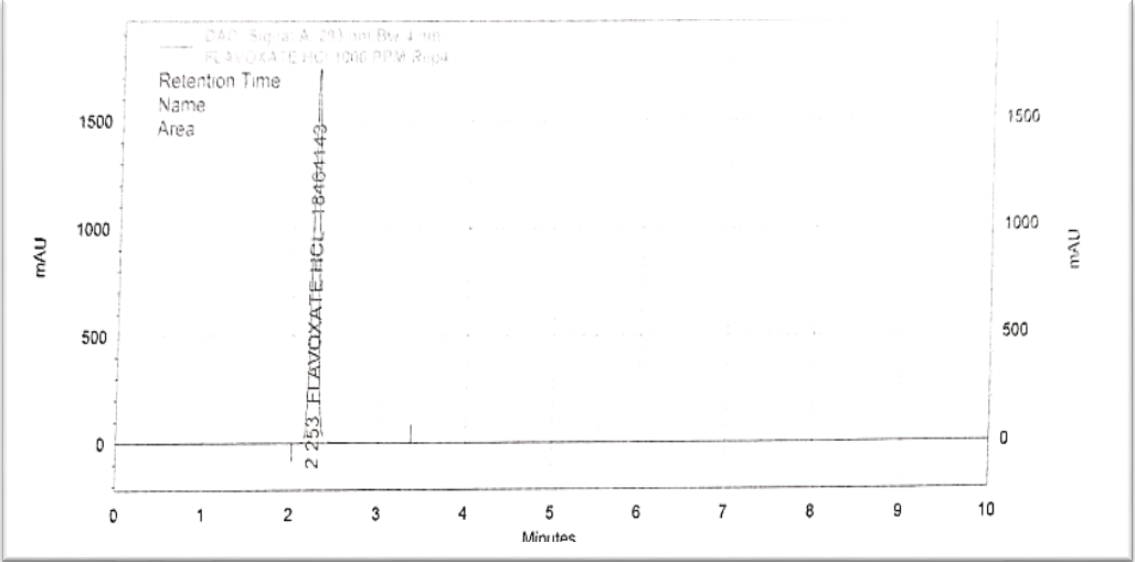
Fig. 3: Chromatogram of flavoxate HCl 1000 PPM
Table 2: Results of system appropriateness study
| Drug | Parameter | Attained value | Approval criteria |
| Flavoxate HCl | Theoretical plates | 4827 | >2000 |
| Retention time | 2.43 | ≥2 | |
| Tailing factor | 1.13 | ≤2 | |
| %RSD Area | 1.99 | ≤2 |
Accuracy
In HPLC, accuracy is the degree to which the measured results closely resemble the actual or recognized reference values of the analyte under investigation. It guarantees the reliability of the results from an HPLC analysis and is a crucial component of technique validation. We have used concentrations about 800-1200 ppm shown in table 3 and fig. 1 to fig. 5 showing concentration from 800-1200 ppm.

Fig. 4: Chromatogram of flavoxate HCl 1100 PPM
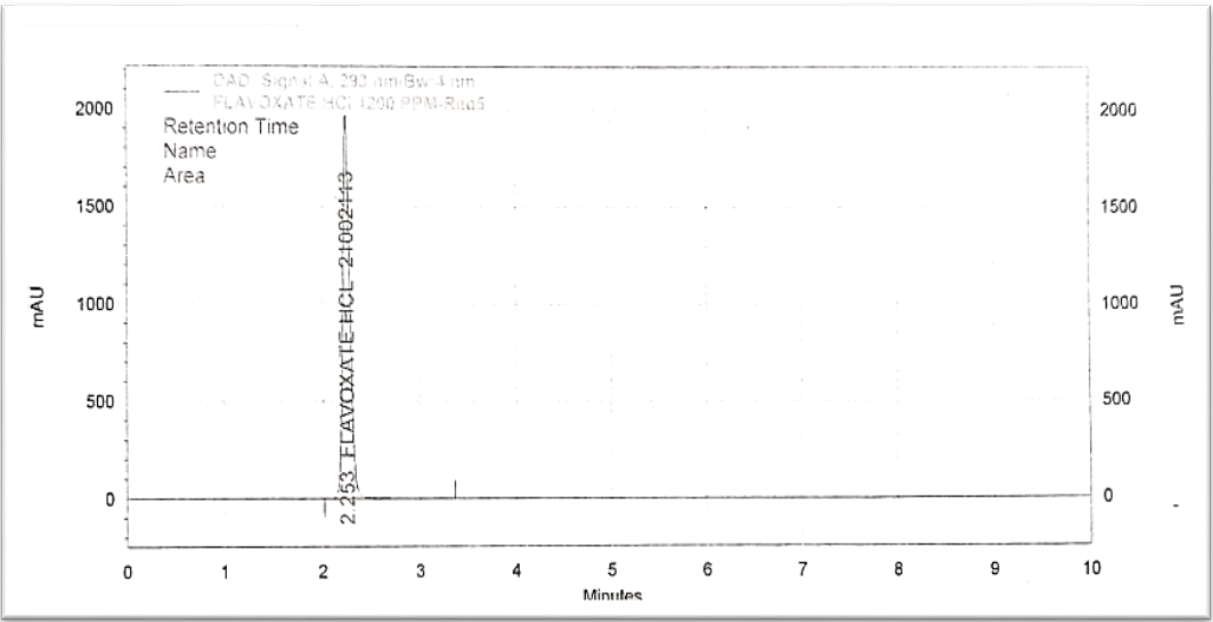
Fig. 5: Chromatogram of flavoxate HCl 1200 PPM
Table 3: Result of accuracy of flavoxate HCl (800-1200)
| S. No. | PPM | ||||
| 800 | 900 | 1000 | 1100 | 1200 | |
| 1 | 14295266 | 15991797 | 18405684 | 19793962 | 20948957 |
| 2 | 14384376 | 16008168 | 18613316 | 19779387 | 20972393 |
| 3 | 14329198 | 15988053 | 18504701 | 19825449 | 20974344 |
| 4 | 14281836 | 16033683 | 18464143 | 19769447 | 20978450 |
| 5 | 14256505 | 16063508 | 18472816 | 19917734 | 21002113 |
| 6 | 14284132 | 16181418 | 18483629 | 19824286 | 20986461 |
| AVG | 14305218.83 | 16044437.83 | 18490714.83 | 19818377.5 | 20977119.67 |
| STD | 45396.83987 | 72813.06384 | 68605.45334 | 53790.70577 | 17532.13338 |
| %RSD | 0.32 | 0.45 | 0.37 | 0.27 | 0.08 |
*Mean±SD (n=6), SD (Standard deviation), %RSD (Percentage relative standard deviation)
Linearity
The link between an analyte's concentration in a sample and the area or height of its corresponding peak in the chromatogram is known as linearity in HPLC, presented in table 4. A linear response means that the peak area (or height) rises in proportion to the analyte's concentration. A fig. 6 represents Linearity graph of flavoxate HCl API.
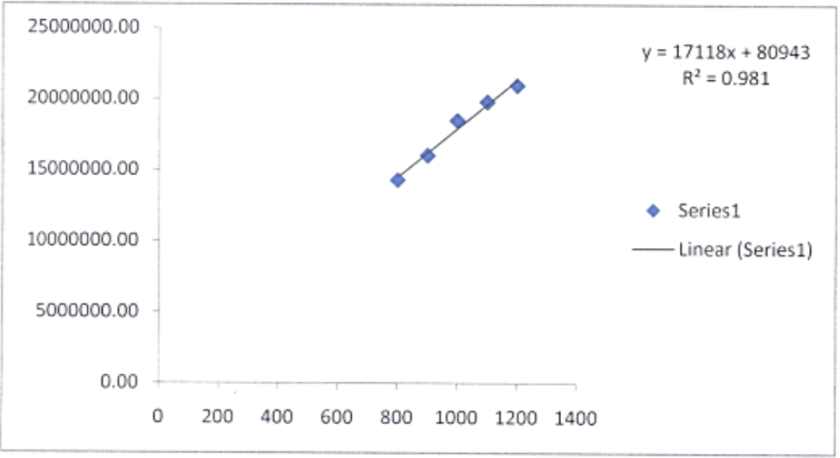
Fig. 6: Appropriate dosage line for flavoxate HCl
Table 4: Result of concentration of flavoxate HCl and the area of its corresponding peak
| PPM | AREA |
| 800 | 14305218.83 |
| 900 | 16044437.83 |
| 1000 | 18490714.83 |
| 1100 | 19818377.50 |
| 1200 | 20977119.67 |
A study on regression analysis, there is a 0.981 association between the mean surface area of the graph and the standard concentration of the drug, indicating that the standard curve is fairly linear. Additional evidence from the regression investigation supports the regression line's linearity and the optimal line's range of 800 to 1200µg/ml.
Precision
In HPLC, precision is the degree of agreement between measurements made repeatedly under the same circumstances. It displays the analytical method's dependability and reproducibility. Especially in regulated industries like pharmaceuticals, precision is essential to guaranteeing that the data produced is reliable and consistent.
Repeatability
Repeatability was considered by analyzing 800 to 1200 PPM attention 6 times a day shown in table 5 and the concentration graph is shown in fig. 7.
Table 5: Result of repeatability (Intra day precision 800-1200 PPM)
| Repeatability (Intra day precision) | |||||
| S. No. | 800 | 900 | 1000 | 1100 | 1200 |
| 1 | 14780223 | 16435531 | 19034754 | 20195201 | 21780580 |
| 2 | 14760495 | 16406116 | 19133439 | 20227602 | 21901590 |
| 3 | 14810197 | 16611877 | 19127131 | 20233586 | 21641135 |
| 4 | 14852538 | 16482782 | 19101582 | 20274640 | 21720767 |
| 5 | 14814247 | 16369946 | 19086423 | 20241127 | 21879022 |
| 6 | 14834352 | 16458447 | 19111010 | 20267069 | 21656812 |
| Mean | 14808675 | 16460783 | 19099057 | 20239871 | 21763318 |
| SD | 33904.17 | 83900.315 | 35816.24 | 28759.8 | 110351.6 |
| %RSD | 0.23 | 0.51 | 0.19 | 0.14 | 0.51 |
*Mean± SD (n=6) SD (Standard deviation), %RSD (Percentage relative standard deviation)
Fig. 7: Chromatogram of precision (Repeatability)
Intermediate precision
The difference in outcomes from different days or from different analysts using the same apparatus and technique. This takes into consideration differences in operator technique, instrument performance, and ambient factors. The concentration used from 800-1200 ppm shown in table 6 and the concentration graph is shown in fig. 8.
Table 6: Result of intermediate precision 800-1200 PPM
| Day 1 | Intermediate precision (Inter day precision) | |||||
| 800 | 900 | 1000 | 1100 | 1200 | ||
| 1 | 14654072 | 16818525 | 18809174 | 20332373 | 22097277 | |
| 2 | 14772280 | 17286618 | 18906626 | 20377498 | 22216379 | |
| 3 | 14854743 | 17442650 | 18922833 | 20437860 | 22171919 | |
| 4 | 15764847 | 17317269 | 19082992 | 20446278 | 22129356 | |
| 5 | 14798846 | 17196773 | 19214123 | 20573082 | 22174336 | |
| 6 | 14816655 | 17380718 | 19170614 | 20552289 | 22130904 | |
| Mean | 14777997.17 | 17240425.5 | 19017727 | 20453230 | 22153361.83 | |
| SD | 68336.28921 | 222944.3774 | 161893.793 | 94660.97914 | 42382.99244 | |
| % RSD | 0.46 | 1.29 | 0.85 | 0.46 | 0.19 | |
| Day 2 | Intermediate precision (Inter day precision) | |||||
| 800 | 900 | 1000 | 1100 | 1200 | ||
| 1 | 16240356 | 16882175 | 20106356 | 20660714 | 21953731 | |
| 2 | 16138631 | 16809622 | 20201355 | 20647943 | 21233547 | |
| 3 | 16212177 | 16754237 | 20195729 | 20665528 | 21424355 | |
| 4 | 16238301 | 16874692 | 20171360 | 20664743 | 21452065 | |
| 5 | 16225553 | 16775753 | 20257830 | 20675665 | 21325889 | |
| 6 | 16155180 | 16848250 | 20222885 | 20690029 | 21365152 | |
| Mean | 13887285.43 | 14420804.14 | 17307930.71 | 17715103.14 | 18393705.57 | |
| SD | 6123495.892 | 6358762.783 | 7631782.61 | 7811151.993 | 8113654.473 | |
| % RSD | 0.27 | 0.31 | 0.25 | 0.07 | 1.19 | |
*Data are given as mean±SD (n=6), % RSD values lower than 2% indicate acceptable precision of the developed method

Fig. 8: Chromatogram of precision (Intermediate)
Robustness
The capacity of HPLC to withstand minor changes in ambient factors and operational settings is known as robustness. In analytical chemistry, this quality is essential for guaranteeing consistent and trustworthy results. Parameters like Mobile Phase, pH, Flow Rate, Wavelength etc., with their number of injections, are shown in table 7.
Table 7: Method for strength
| Parameter | Injection |
| Mobile Phase Composition (±10%) | 01 injection of blank 04 injection of mixed standard solution |
| pH (± 0.05) | 01 injection of empty 04 injection of combined standard solution |
| Flow Rate (±10%) | 01 injection of empty 04 injection of combined standard solution |
| Wavelength (±2 nm) | 01 injection of empty 04 injection of combined standard solution |
| Column Temperature (±10%) | 01 injection of empty 04 injection of combined standard solution |
Force degradation study
The degradation condition as per ICH guidelines Q1A (R2). Reagent strength and exposure time were two factors that were optimized to achieve a 10-30% degradation. The following are the ideal circumstances. Procedure for force degradation is shown in table 8 and the concentration graph is shown in fig. 9 to fig. 13 [20-22].
Table 8: Procedure for force degradation
| Acidic degradation | Add5 M 5 ml HCl in mixed test solution+stand for 15 min+neutralize with same concentration of alkali | 01 injection of blank 01 injection of std. solution 06 injection of test solution |
| Alkali degradation | Add 5M 5 ml NaOH in mixed test mixture+stand for 15 min+neutralize with same concentration of acid. | 01 injection of blank 01 injection of std. result 06 injection of test solution |
| Oxidative degradation | Add 2%-5 ml and 10 ml H2O2, add 3%-5 ml and 10 ml H2O2 | 01 injection of blank 01 injection of std. solution 06 injection of test solution |
| Thermal degradation | Treated at increased temperature (60 °C) for 24 h | 01 injection of blank 01 injection of std. solution 06 injection of test solution |
| Photolytic degradation (Light) | Light exposure of sample-24 h | 01 injection of blank 01 injection of std. solution 06 injection of test explanation |
| Photolytic degradation (UV) | UV exposure of sample-24 h | 01 injection of blank 01 standard solution injection 06 Test answer injection |
Fig. 9: Chromatogram of acid degradation
Fig. 10: Chromatogram of alkali degradation

Fig. 11: Chromatogram of oxidative degradation
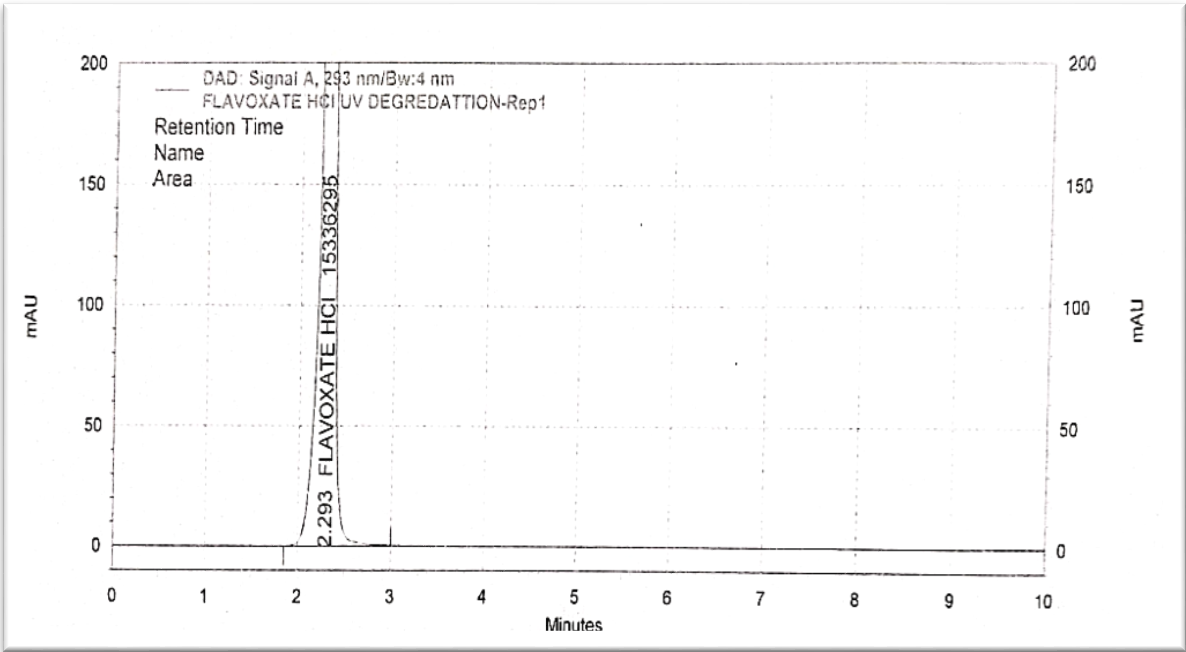
Fig. 12: Chromatogram of photolytic degradation
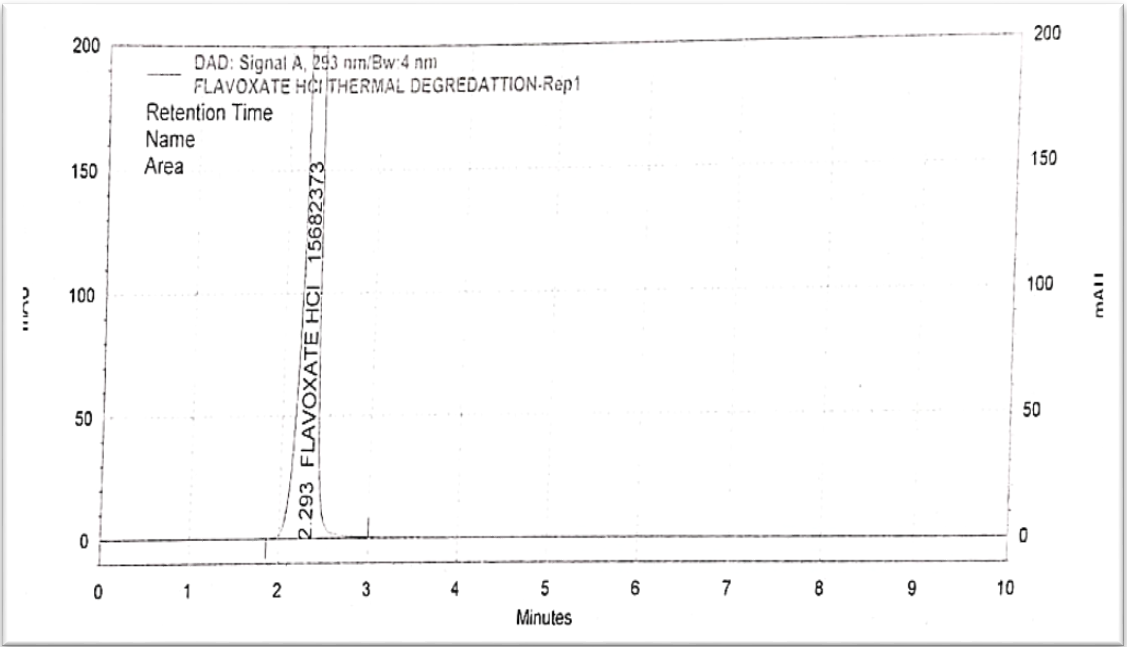
Fig. 13: Chromatogram of thermal degradation
IR spectrum analysis
Infrared spectroscopy (IR) is a method for studying how molecules react to infrared light. Three distinct types of measurements, absorption, emission, and reflection, can be used to investigate this. Inorganic and organic chemists rely on this technique the most. It is used by chemists to determine which functional groups are present in compounds [21]. Infrared Spectra verify its chemical makeup 2-piperedin-1-ylethyl 3-methyl-4-oxo-2-phenylchromene-8-carboxylate it is. Fig. 14 displays the infrared spectrum.
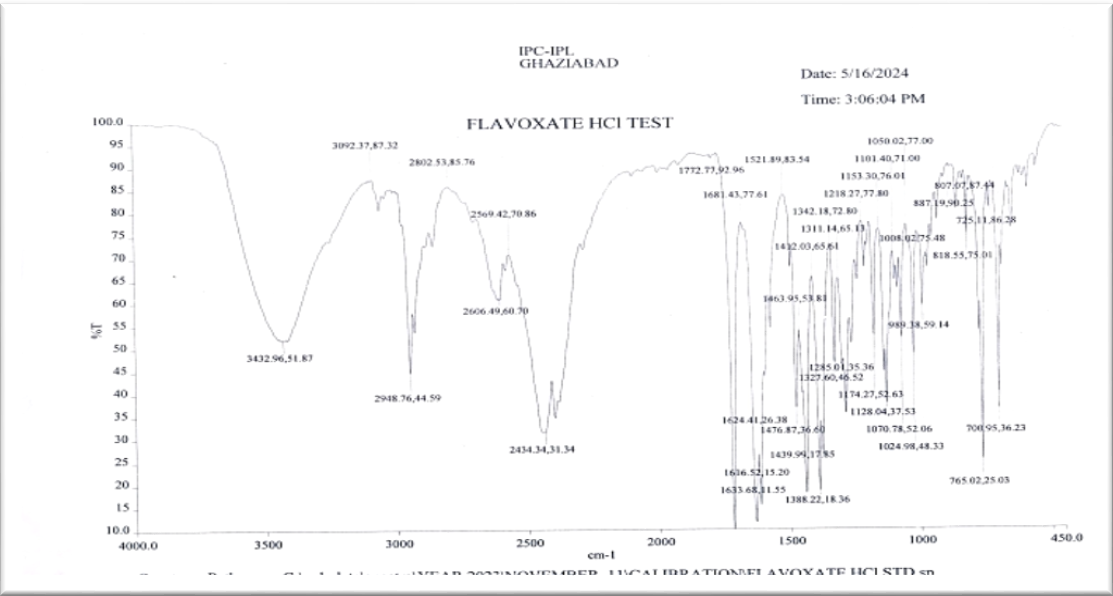
Fig. 14: IR spectrum of flavoxate HCl API
Mass spectrometry
A key analytical method for studying proteins and biomolecules in general is mass spectrometry. Numerous new mass spectrometry-based analytical systems and experimental techniques have been developed in response to the need to identify, describe, and quantify proteins in ever-more complex samples with ever-increasing sensitivity. In mass spectrometry (MS), ionised sample molecules are separated using electronic and/or magnetic fields according to their form-to-charge ratios (m/z), a sensitive and potent analytical method. It is shown in fig. 15 [22].

Fig. 15: Mass spectrometer of flavoxate HCl API
NMR spectroscopy
For figuring out the structure of organic substances, NMR spectroscopy is a potent method that may be used to examine the dynamics, structure, and chemical kinetics of a variety of many biochemical systems. Becoming a practicing NMR spectroscopist can be difficult for a newbie due to the intricacy of this approach. The main purpose of this book is to offer a theoretical and practical introduction to contemporary NMR spectroscopy. It seems to be shown in fig. 16 [23].
UV-visible spectroscopy
UV-Vis spectroscopic scanning is a simple, non-intrusive analytical method that has the potential to support formulation development mechanistically. Over the past five years, UV scanning has shown promise in a variety of drug substances and delivery methods, and it is also useful both qualitatively and quantitatively in some more specialized research. The simplest way to run various analyses is to use the principle of UV-Spectroscopy. Samples were recorded from 200 to 400 nm, and after the linearity analysis, the λmax was confirmed to be 293 nm. It seems to be shown in the fig. 17 [24, 25].
In comparison to current HPLC methods for pharmaceutical analysis, this study successfully developed and validated a novel and effective reverse-phase high-performance liquid chromatography (RP-HPLC) method for the quantification of flavoxate HCl. The suggested approach offers significant advantages in terms of solvent consumption and time efficiency. Retention period of flavoxate HCl was 2.43 min is significantly less than that of earlier investigations, which reduces analysis time and boosts laboratory productivity. Another significant benefit of the approach is its reduced solvent usage, which lowers operating costs and environmental impact, two factors that are crucial in green analytical chemistry. Excellent linearity is suggested by the correlation coefficient (R2=0.9981) derived from linear regression analysis, demonstrating the method's dependability at various flavoxate HCl concentrations. The good peak resolution and significant absorbance at a wavelength of 293 nm further increase the method's sensitivity. In order to ensure the safety and effectiveness of pharmaceutical products containing this API, this degree of sensitivity is especially helpful for detecting tiny levels of flavoxate HCl. The suggested method's speed, ease of use, and solvent efficiency make it a very appealing choice for the pharmaceutical business, even though other HPLC techniques might yield trustworthy results. The continuous %RSD values and the strict respect to ICH criteria for validation parameters proved the method's remarkable robustness, accuracy, and precision. The method's specificity is further supported by the outstanding peak separation and resolution, which showed no interference from excipients or other possible contaminants. The pharmaceutical firms that produce flavoxate HCl or comparable substances can profit greatly from the developed RP-HPLC technology. It is ideal for routine quality control testing because of its quick analysis time, which enables the effective processing of huge sample volumes.

Fig. 16: NMR spectroscopy of flavoxate HCl API

Fig. 17: UV-visible spectroscopy of flavoxate HCl API
CONCLUSION
RP-HPLC equipment was utilized in the improvement and authorization of a fast and precise method for measuring and testing the drug flavoxate HCl. Although this method was straightforward, it was original and had never been published before. According to ICH guidelines, a sensitive, straightforward and cost-effective HPLC technique with a PDA detector was created and validated in this work for the detection of flavoxate HCl. The process was simple, accurate, efficient, and trustworthy. Additionally, it was found to comply with the ICH requirements for robustness, accuracy, precision, linearity, specificity, and system applicability. Therefore, flavoxate HCl can be routinely analyzed using this approach. Because of its accurate % RSD values, linear curve, excellent peak separation, and enhanced resolution, this method is recommended for detecting flavoxate HCl.
LIST OF ABBREVIATIONS
RP-HPLC: Reverse phase high-performance liquid chromatography; OPA: Orthophosphoric Acid; TMA: Triethylamine; ACN: Acetonitrile; UV: Ultraviolet; LOD: Limit of Detection; LOQ: Limit of Quantitation; OAB: Overactive Bladder; BBB: Blood Brain Barrier; ICH: International Conference on Harmonization; API: Active Pharmaceutical Ingredient; IR: Infrared; MS: Mass Spectrometry; NMR: Nuclear Magnetic Resonance.
ACKNOWLEDGEMENT
The Almighty God, is the first and foremost to be praised and thanked for his many mercies in helping me finish my dissertation successfully. Without acknowledging the individuals who enabled the effective completion of any task their unwavering leadership, support, and encouragement that culminates in the success of all efforts. Happiness that comes with its accomplishment would be lacking.
My research supervisor at Sanskar Educational Group in Uttar Pradesh, India, Prof. (Dr.) Shabnam Ain, Head of Department-College of Pharmacy, has been an inspiration to me throughout this research project, and for that I am truly grateful. Her creative advice, energy, vision, genuineness and moral support enabled me to finish my research project to the best of my ability. I will always be grateful to Director Prof. (Dr.) Babita Kumar for all the facilities she provided for my research work and Dr. Qurratul Ain for his constructive critique and artistic recommendations.
The authors thank the Indian Pharmacopoeial Commission (IPC), Ghaziabad for providing the necessary laboratory facilities. Furthermore, the authors extend their gratitude to Principal Scientific Officers. Dr. Robin Kumar and Dr. Meenakshi Dahiya, IPC for their tireless efforts and guidance, which have culminated in this thesis.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
All of the writers made an equal contribution to the study project. Aakriti Bhardwaj contributed by gathering the chemicals and reagents needed for our laboratories' experimental work and by carrying out the essential tasks. Dr. Shabnam Ain contributed by evaluating and interpreting the information. Dr. Babita Kumar provided the facilities for my research work. By carrying out the task under his supervision, Dr. Robin Kumar provided assistance. The manuscript was written by Dr. Qurratul Ain, who also made revisions to ensure its excellence.
CONFLICT OF INTRESTS
The authors state that they have no conflict of interest
REFERENCES
Yadav AV, Gaud RS. Pharmaceutics. 10th ed. Pragati Books Pvt. Ltd; 2016. p. 1.
Sharma S, Goyal S, Chauhan K. Analytical method development and validation. Int J App Pharm. 2018;10(6):8-15. doi: 10.22159/ijap.2018v10i6.28279.
Ain S, Ain Q, Kumar B. Investigations on novel bio-binder isolated from Aegle marmelos used in ibuprofen tablets. Int J Pharm Pharm Sci. 2015;7(7):414-5.
Henderson E, Drake M. Overactive bladder. Maturitas. 2010 Jul;66(3):257-62. doi: 10.1016/j.maturitas.2010.03.010, PMID 20399043.
Wallace KM, Drake MJ. Overactive bladder. F1000Research. 2015;4:F1000 Faculty Rev-1406. doi: 10.12688/ f1000research.7131.1, PMID 26918151.
Ain S, Philip B, Pathak K. Preformulative assessment of preformed complexes of gemfibrozil, with cyclodextrins. PDA J Pharm Sci Technol. 2008;62(4):300-8. PMID 19174958.
Ain S, Kumar B, Pathak K. Development and characterization of controlled release famotidine matrix tablets containing complexes. Int J Appl Pharm. 2017;9(4):8-46. doi: 10.22159/ijap.2017v9i4.18859.
Sweeney P, Mutambirwa S, Van An N, Sharma JB, Vanamail P. Flavoxate in the symptomatic treatment of overactive bladder: a meta-analysis. Eur Rev Med Pharmacol Sci. 2016;20(17); Sep:3703-12. PMID 27649675.
Fehrmann-Zumpe P, Karbe K, Blessman G. Using flavoxate as primary medication for patients suffering from urge symptomatology. Int Urogynecol J Pelvic Floor Dysfunct. 1999 Apr;10(2):91-5. doi: 10.1007/pl00004018, PMID 10384969.
Bhardwaj SK, Dwivedia K, Agarwala DD. HPLC method development and validation. Int J Anal Bioanal Chem. 2015; Nov;5(4):76-81.
El-Shaheny RN, El-Enany NM, Belal FF. A green HPLC method for the analysis and stability study of flavoxate HCl using micellar eluent. Anal Methods. 2014;6(4):1001-10. doi: 10.1039/C3AY41318G.
Snyder LR, Glajch JL, Kirkland JJ, Abbott RW. Practical HPLC method development. Analytica Chimica Acta. 1991;245:287-8. doi: 10.1016/S0003-2670(00)80235-4.
Guideline, ICH harmonised tripartite. Validation Anal Proc Text Methodol. 2005;Q2(R1):1.20.
Jadhav S, Bhoir G, Jain V. Method development and validation of rapid isocratic RP-HPLC method for simultaneous estimation of paracetamol, caffeine, and propyphenazone in pharmaceutical formulation. Asian J Pharm Clin Res. 2024;17(4):83-7. doi: 10.22159/ajpcr.2024.v17i4.49544.
Guideline, IHT. Stability testing of new drug substances and products, Q1A(R2). Current Step. 2003;4:1-24.
Ain S, Gupta V, KB, Ain Q, Dahiya J. Solubility enhancement of the poorly water-soluble antiulcer drug famotidine by inclusion complexation. PCI- Approved-IJPSN. 2013;6(1):1983-9. doi: 10.37285/ijpsn.2013.6.1.10.
Kumar V, Ain S, Kumar B, Ain Q, Gaurav. Optimization and evaluation of topical gel containing solid lipid nanoparticles loaded with luliconazole and its antifungal activity. Int J Pharm Res. 2020;12(2):2901-12.
Dhama PK, Ain S, Kumar B, Ain Q. Development and evaluation of topical ointment formulation containing gallic acid as an active pharmaceutical ingredient against bacterial infection and oxidative damage. AP. 2022;11(1):439-49. doi: 10.54085/ap.2022.11.1.51.
Guideline, ICH harmonised tripartite. Stability testing: photostability testing of new drug substances and products. Vol. Q1B. Geneva: ICH; 1996. p. 172.
ICH. Stability testing of new drug substances and products. International Conference on Harmonization; Geneva Q1A (R2); 2003.
Stuart BH. Infrared spectroscopy: fundamentals and applications. John Wiley & Sons; 2004. doi: 10.1002/0470011149.
Domon B, Aebersold R. Mass spectrometry and protein analysis. Science. 2006;312(5771):212-7. doi: 10.1126/science.1124619, PMID 16614208.
Rule GS, Kevin Hitchens T. NMR spectroscopy. Springer Netherlands; 2006.
Parveen N, Routh T, Goswami AK, Mondal SA. New robust analytical method development, validation, and stress degradation studies for estimating ritonavir by UV-spectroscopy and HPLC methods. Int J App Pharm. 2023;15(4):214-24. doi: 10.22159/ijap.2023v15i4.47924.
Ali Z, Ain S, Kumar B, Ain Q. Method development and validation for estimation of cefadroxil in different marketed tablets by UV spectrophotometry method and anti-inflammatory studies using in-silico approaches. Orient J Chem. 2022;38(4):898-905. doi: 10.13005/ojc/380409.