
Int J Pharm Pharm Sci, Vol 17, Issue 3, 10-15Original Article
A STUDY TO EVALUATE THE EFFECT OF EMPAGLIFLOZIN ON CARDIOVASCULAR AND RENAL OUTCOMES OF PATIENTS WITH TYPE 2 DIABETES MELLITUS AT A TERTIARY CARE HOSPITAL IN TELANGANA
K. VASANTHA KUMARI, MEHER KOUNEN FATIMA, FARHEEN SULTANA*, CHAKRADHAR T., MOHD MOOSA QUADRI
Department of Pharmacology, Osmania Medical College, Koti, Hyderabad, India
*Corresponding author: Farheen Sultana; *Email: malekabegum63@gmail.com
Received: 14 Nov 2024, Revised and Accepted: 17 Jan 2025
ABSTRACT
Objective: The objective of this study is to evaluate the effects of Empagliflozin on cardiovascular and renal outcomes in Type 2 Diabetes Mellites (T2DM) patients, particularly focusing on improvements in glycemic control, weight, blood pressure, and renal function after 6 mo of treatment.
Methods: A cross-sectional observational study was conducted at Osmania Medical College and Hospitals, Hyderabad, over six months, involving 120 patients with T2DM. The patients were randomly divided into two groups. Group 1 patients received standard Oral Hypoglycemic Agents (OHAs) such as Metformin. Group 2 patients received the same OHAs along with Empagliflozin 10 mg. Baseline and post-treatment data for key parameters such as blood glucose levels, blood pressure, Body Mass Index (BMI), and renal function markers (e. g., serum creatinine) were collected. The t-test was used for statistical analysis to compare pre-and post-treatment values.
Results: After six months of treatment, the following significant improvements were observed in the group receiving Empagliflozin: Diastolic blood pressure: A significant reduction of 7 mmHg (p = 0.00066), BMI: A reduction of 1.6 units (p = 0.0107), Key glycemic parameters showed significant improvements: Fasting Blood Sugar (FBS): p = 0.000212, Random Blood Sugar (RBS): p<0.00001, HbA1c levels: p = 0.000147. These results indicate that Empagliflozin significantly improved glycemic control, blood pressure, and weight, which are all key factors contributing to improved cardiovascular and renal health in T2DM patients.
Conclusion: The addition of Empagliflozin to standard T2DM treatment resulted in significant improvements in glycemic control, body weight, and blood pressure, particularly diastolic blood pressure, over the 6-month study period. These changes are likely to contribute to improved cardiovascular and renal outcomes in patients with T2DM. Thus, Empagliflozin offers a valuable therapeutic option for enhancing overall disease management in T2DM patients with complications.
Keywords: Type 2 diabetes mellitus, Metformin, Empagliflozin, Cardiovascular outcomes, Renal outcomes
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijpps.2025v17i3.53190 Journal homepage: https://innovareacademics.in/journals/index.php/ijpps
INTRODUCTION
Diabetes mellitus (DM), particularly Type 2 Diabetes Mellitus (T2DM), is one of the leading causes of morbidity and mortality worldwide. The condition significantly contributes to various complications, including cardiovascular disease, kidney failure, neuropathy, and retinopathy, all of which can lead to premature death if not adequately managed. According to World Health Organization (WHO), Non-Communicable Diseases (NCDs) accounted for 74% of deaths globally in 2019, among which Diabetes caused 1.6 million deaths [1]. Thus, T2DM became the 9th leading cause of death globally [2]. The prevalence of Diabetes in India has risen from 7.1% in 2009 to 11.4 in 2023 [3]. Various factors, including lifestyle changes, urbanization, and an aging population, drive the prevalence of T2DM in India. Obesity, high blood pressure (hypertension), and hypertriglyceridemia are common risk factors in people with diabetes [4]. These factors not only accelerate the progression of T2DM but also significantly increase the risk of developing cardiovascular diseases, stroke, nephropathy, retinopathy, neuropathy, and other diabetes-related complications [5]. These complications are often the main contributors to the premature morbidity and mortality observed in diabetic patients. The underlying mechanisms responsible for these complications involve complex biochemical processes such as oxidative stress, which results from the overproduction of Reactive Oxygen Species (ROS) and insulin signaling defects that lead to insulin resistance [6, 7]. Adults with T2DM have a 2-to-4-fold higher risk for cardiovascular morbidity and mortality than adults without diabetes, according to the American Heart Association (AHA) [8]. The treatment of Diabetes in the elderly is challenging concerning the potential drug-drug interactions, paucity of data from randomized trials, and lack of specific guidelines [9]. Metformin is the preferred medication because it is more efficacious in reducing HbA1c levels by 1.5 to 2%, Fasting blood glucose by 60-80 mg/dl, plasma triglycerides, and Low-Density Lipoprotein (LDL) levels by 8-15% [10, 11]. The progressive nature of Type 2 Diabetes means that beta-cell function gradually declines over time, leading to a need for additional medications beyond Metformin to maintain optimal glycemic control. By using a combination of oral hypoglycemic agents or adding insulin therapy, healthcare providers can help patients achieve and maintain target blood glucose levels, thus reducing the risk of complications like cardiovascular disease, kidney damage, and neuropathy. Regular re-evaluation and adjustment of the treatment regimen are key to managing this chronic, progressive disease effectively. Also, new anti-hyperglycemic agents, such as Sodium Glucose Transporter-2 Inhibitors (SGLT-2 inhibitors), have the potential to address the unmet needs associated with conventional anti-hyperglycemic agents, including the improvement of glycemic control with the modification of cardiovascular and renal factors [12]. Because the mechanism of action is independent of beta cell function and insulin pathway, the risk of hypoglycemia is less with SGLT-2 inhibitors. Empagliflozin is a highly potent, competitive inhibitor of SGLT-2 and has been approved as a treatment for T2DM in patients with normal renal function [13]. Empagliflozin offers the convenience of once-daily oral administration when compared with other members of this group of drugs. Besides, lowering Empagliflozin exerts a favorable effect on several non-glycemic outcomes, including modest reductions in body weight and blood pressure [14].
The study aims to evaluate the short-term effect of empagliflozin on cardiovascular and renal outcomes of patients with type 2 Diabetes mellitus.
MATERIALS AND METHODS
Study design
This study is a cross-sectional observational study aimed at evaluating the short-term effect of empagliflozin on cardiovascular and renal outcomes in patients with T2DM. The study was approved by the Institutional Ethics Committee of Osmania Medical College before commencement (IEC/OMC/2022/M. NO. (03), Acad-14).
Study setting
The study was conducted at Osmania General Hospital, a tertiary care teaching hospital located in Hyderabad, Telangana. The study was conducted over 6 mo, from March 2023 to August 2023, during which patients with T2DM and complications were evaluated and followed up.
Study duration
The total duration of the study was 6 mo.
Study subjects
The study included T2DM patients with cardiovascular and renal impairment. These patients were selected to assess the effects of Empagliflozin 10 mg on cardiovascular and renal outcomes.
Study tool
The study evaluated the patients based on their blood sugar levels, including HbA1c (Glycated Hemoglobin), FBS, and Postprandial Blood Sugar (PLBS) levels. The collected data was analyzed statistically to determine the effects of Empagliflozin on both cardiovascular and renal outcomes in these patients.
Inclusion criteria
Patients meeting the following criteria were included: Diagnosed with type 2 Diabetes Mellitus, HbA1c>6%, and currently on oral hypoglycemic agents, age between 25 to 85 y, presenting with cardiovascular and renal impairment (eGFR>60 ml/min), willing to provide informed consent for participation in the study.
Exclusion criteria
Pregnant or lactating women, patients with type 1 diabetes mellitus or other types of diabetes, non-compliant patients, or those with renal failure of non-diabetic etiology (eGFR<60 ml/min), and patients who were unwilling to provide informed consent were excluded from the study.
Sample size
The study initially evaluated 130 T2DM patients, out of which 120 patients were included in the study. The exclusion of the remaining 10 patients occurred for the following reasons:
3patients had chronic kidney disease of non-diabetic etiology, 6 patients dropped out by not showing up for the study visits, and 1 patient declined to give informed consent during the study period.
Detailed history and physical examination
A comprehensive history was taken for each patient, including details about present and past medical history, family history of diabetes or other diseases, dietary habits, and drug history (current medications and treatments). A thorough general physical and systemic examination was conducted for each patient.
Grouping of patients
After meeting the inclusion criteria, 120 patients were randomly allocated into 2 groups (Group 1 and Group 2) using computer-generated randomization.
Group-1: Patients of group 1 were treated with metformin 500 mg.
Group-2: Patients in this group continued their routine oral hypoglycemic agents with the addition of SGLT-2 inhibitor Empagliflozin 10 mg.
Baseline investigations
The baseline investigations such as Fasting Blood Sugar (FBS), Random Blood Sugar (RBS), HbA1c levels, Body Mass Index (BMI), Systolic and Diastolic Blood Pressure (SBP and DBP), Lipid Profile (Total cholesterol, Triglycerides, LDL, HDL), Serum Creatinine levels were performed for all patients.
Treatment and follow-up
Based on their group allocation, patients were administered the corresponding treatment (Metformin therapy for group 1 and Metformin+Empagliflozin for group 2). Follow-up was done for 6 mo, during which the treatment efficacy of Empagliflozin on cardiovascular and renal outcomes was assessed. The post-treatment parameters were compared with the baseline data to determine the effect of Empagliflozin on the primary outcomes of interest.
Fig. 1: Methodology of the study
Statistical analysis
The collected data was analyzed using Statistical Package for the Social Sciences (SPSS) software version 25. T-tests were used to compare the pre-treatment and post-treatment parameters within each group and between the two groups. Statistical significance was defined by a p-value<0.05.
Outcome measures
The primary endpoints of the study focused on glycemic control (FBS, RBS, HbA1c) and blood pressure management (systolic and diastolic BP), which are critical factors in evaluating cardiovascular and renal outcomes in T2DM. The secondary endpoints (BMI, lipid profile parameters) were important for understanding the broader effects of Empagliflozin 10 mg on cardiometabolic health, including weight loss and improvement in lipid metabolism. The study aimed to provide comprehensive evidence on how Empagliflozin can positively influence both glycemic control and cardiovascular/renal health in diabetic patients.
RESULTS
A total of 120 patients participated in the study, which was conducted over 6 mo. The patients were categorized into Group 1 and Group 2. The following are the key demographic and baseline characteristics of the study population:
Age Distribution: Age Range: 25 to 85 y, mean Age: 55 y. Gender Distribution: Male: 78 patients (65%), Female: 42 patients (35%). Age Group Distribution: 85% of patients were aged between 40-75 y. Weight and Body Mass Index (BMI): Weight Range: 78 kg to 86 kg, mean Weight: 82 kg. BMI Distribution: 66.6% of patients (80 out of 120) had a BMI between 25 and 30 (overweight). 33.3% of patients (40 out of 120) had a BMI above 30 (obese). Hypertension: Hypertensive Patients: 76 out of 120 (63.3%). Non-hypertensive Patients: 44 out of 120 (36.6%). Glycemic Control (HbA1c Levels): HbA1c<7%: 39 out of 120 (32.5%), HbA1c between 7-8%: 72 out of 120 (60%), HbA1c between 8-9%: 9 out of 120 (7.5%). Lipid Profile (Baseline Levels): Total Cholesterol, Triglycerides, and LDL: There was a slight decrease observed in the levels of total cholesterol, triglycerides, and LDL cholesterol throughout the study. HDL (Good Cholesterol): A slight increase in HDL levels was observed during the follow-up period.
These baseline characteristics provide an overview of the study population, highlighting a predominance of middle-aged patients with a high prevalence of hypertension and overweight/obesity. The patients generally exhibited moderate to poor glycemic control at the beginning of the study, with a majority having HbA1c between 7-8%. The slight improvements in lipid profiles and BMI during the study suggest that Empagliflozin may have had beneficial effects on both cardiovascular and renal outcomes in this cohort of patients with Type 2 Diabetes Mellitus. The baseline characteristics of the study population are shown in table 1.
The baseline and post-treatment parameters were evaluated within each group using a paired t-test to determine the significance of changes over the 6-month treatment period. The results of this analysis are summarized in table 2, showing significant improvements in several key health parameters for both Group 1 (patients on oral hypoglycemic agents) and Group 2 (patients on oral hypoglycemic agents plus Empagliflozin).
Table 1: Baseline characteristics
| Factors | Mean | SD |
| Age group(25-85y) | 55 | 6.38 |
| Male to female ratio | 78:42 | 0.93 |
| Duration of diabetes (y) | 4 | 0.85 |
| Weight (kg) | 82.5 | 5.52 |
| BMI | 25.84 | 4.15 |
| SBP (mm Hg) | 141.4 | 19.83 |
| DBP (mm Hg) | 83.8 | 10.11 |
| FBS (mg/dl) | 134.5 | 3.83 |
| RBS (mg/dl) | 166.9 | 4.27 |
| HbA1c (n %) | 7.35 | 0.85 |
| Total Cholesterol | 213 | 23.03 |
| Triglyceride (TG) | 164.4 | 30.06 |
| LDL | 136.4 | 18.51 |
| HDL | 43.5 | 6.51 |
| Serum Creatinine | 2.1 | 0.51 |
BMI-Body Mass Index, SBP-Systolic Blood Pressure, DBP-Diastolic Blood Pressure, FBS-Fasting Blood Sugars, RBS-Random Blood Sugars, LDL-low density lipoprotein, HDL-High Density Lipoprotein.
Table 2: Comparision of the baseline and post-treatment results
| Group-1 | Group-2 | |||||
| Parameter | Baseline average | Post-treatment average | P-value | Baseline average | Post-treatment average | P-value |
| SBP (mm Hg) | 143.2 | 140.6 | 0.00041 | 139.6 | 131.6 | 0.00001 |
| DBP (mm Hg) | 83.83 | 82.3 | 0.0002 | 83.93 | 77 | 0.0001 |
| BMI | 26.2 | 25.28 | <0.00001 | 25.48 | 23.68 | <0.00001 |
| FBS (mg/dl) | 134.36 | 125.81 | <0.00001 | 134.65 | 124.18 | <0.00001 |
| RBS (mg/dl) | 166.91 | 138.8 | <0.00001 | 167 | 136.01 | <0.00001 |
| HbA1c (n %) | 7.31 | 6.72 | <0.00001 | 7.38 | 6.37 | <0.00001 |
| Total Cholesterol | 211.56 | 201.65 | <0.00001 | 214.5 | 200.9 | <0.00001 |
| Triglyceride | 163.18 | 150.68 | <0.00001 | 165.63 | 138.16 | <0.00001 |
| LDL | 135.65 | 115.71 | <0.00001 | 137.2 | 108.41 | <0.00001 |
| HDL | 41.46 | 43.33 | <0.00001 | 45.7 | 48.93 | <0.00001 |
| Sr. Creatinine | 2.1 | 1.8 | <.00001 | 1.8 | 1.6 | <.00001 |
After following up with the patients for 6 mo, the results of Group 1 (treated with oral hypoglycemic agents) were compared with those of Group 2 (treated with oral hypoglycemic agents in combination with Empagliflozin 10 mg). The findings are summarized below:
Baseline systolic blood pressure showed a modest reduction after treatment in Group 1 patients. A reduction in systolic blood pressure, showing a difference of about 10 mm Hg was observed in Group 2 patients(p =0.001173), which is statistically significant indicating a greater reduction in systolic blood pressure. Graph 2 clearly illustrates this improvement. Group 1 patients showed a slight reduction in diastolic blood pressure after treatment while Group 2 patients demonstrated a greater improvement in diastolic blood pressure, with a difference of about 7 mm Hg between the two groups (p = 0.00066), indicating the superior effect of Empagliflozin in lowering diastolic blood pressure. Patients of Group 1 showed a minor decrease in BMI after 6 mo of treatment. Group 2 patients showed a greater decrease in BMI, with a difference of 1.6 between the two groups (p =0.010699), which is statistically significant, suggesting that Empagliflozin promotes greater weight loss compared to the standard oral hypoglycemic agents. A Significant improvement in Fasting Blood Sugar (FBS), Random Blood Sugar (RBS), and HbA1c was observed in Group 2 (FBS p-value: 0.000212, RBS p-value:<0.00001, HbA1c p-value: 0.000147). All these p-values are less than 0.05, indicating improvements in glycemic control in Group 2 with the addition of Empagliflozin. Total Cholesterol showed no significant difference between the two groups, as the p-value was 0.82. A significant reduction was observed in triglyceride levels in Group 2, (p = 0.001355). There was an increase in HDL levels and a reduction in LDL levels in Group 2 compared to Group 1, with p-values of 0.000065 and<0.00001, respectively, indicating significant improvements in lipoproteins. Baseline Sr. creatinine of Group 1 patients showed a modest reduction after treatment. Significant reduction of Sr. creatinine in Group 2 patients, showing a difference of about 0.2 mg/dl compared to Group 1 (p<0.00001), which is statistically significant, indicating a greater improvement in the Empagliflozin group (Group 2).
The addition of Empagliflozin to the OHAs in Group 2 showed significant improvements in cardiovascular and renal outcomes when compared to the patients treated with Metformin alone (Group 1). Group 2 showed better blood pressure control, glycemic control, and improvements in lipid profile (including triglycerides, HDL, and LDL) as well as a greater reduction in BMI. These significant differences (with p-values<0.05) confirm that Empagliflozin added to the treatment regimen of oral hypoglycemics provided superior clinical outcomes, particularly in terms of cardiovascular health, renal function, and weight loss. The results strongly suggest that Empagliflozin is an effective add-on therapy for patients with Type 2 Diabetes Mellitus, particularly those with cardiovascular and renal complications, enhancing both glycemic and cardiovascular outcomes.
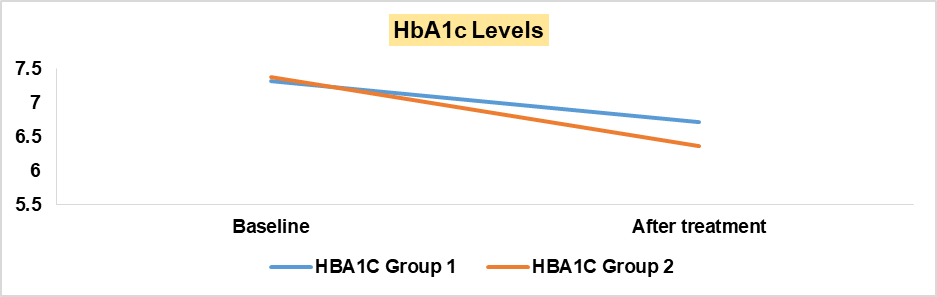
Fig. 2: Comparison of baseline and after-treatment HbA1c levels
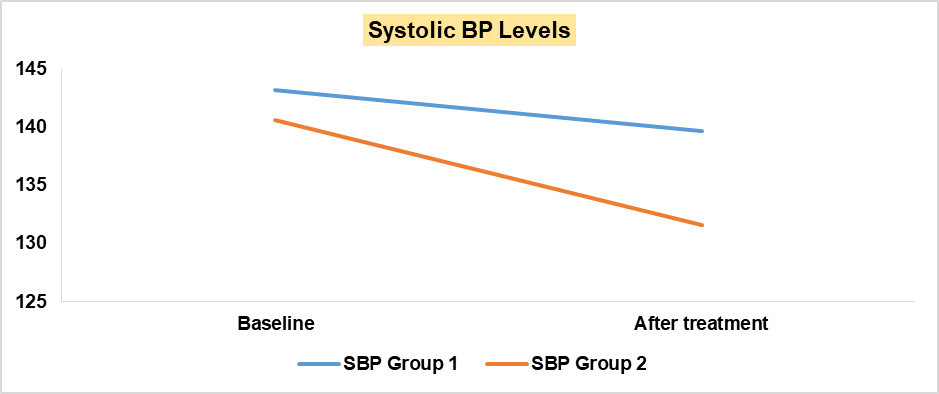
Fig. 3: Comparison of baseline and after-treatment systolic BP levels
Table 3: Comparison of the results of group 1 and group 2 after treatment
| Parameter | Group-1 average | Group-2 average | P-value |
| SBP (mm Hg) | 140.63 | 131.16 | 0.001173 |
| DBP (mm Hg) | 83.2 | 77 | 0.00066 |
| BMI | 25.28 | 23.68 | 0.010699 |
| FBS (mg/dl) | 125.81 | 124.18 | 0.000212 |
| RBS (mg/dl) | 138.8 | 136.01 | <0.00001 |
| HbA1c (n %) | 6.72 | 6.37 | 0.000147 |
| Total Cholesterol | 201.65 | 200.9 | 0.825858 |
| Triglyceride | 150.68 | 138.16 | 0.001355 |
| LDL | 115.71 | 108.41 | 0.000065 |
| HDL | 43.33 | 48.93 | <0.00001 |
| Sr. Creatinine | 1.8 | 1.6 | <0.00001 |
DISCUSSION
In this short-term observational study, there was a statistically significant reduction in glycosylated hemoglobin, fasting blood glucose, body mass index, and favorable lipid profile changes in group 2 patients when compared with group 1 patients. Studies of SGLT-2 inhibitors with a placebo established that SGLT-2 inhibitors were weak glucose-lowering agents showing a modest reduction in HbA1c. These also inhibit both hyper and hypoglycemia by modulating urinary glucose losses, including enhanced lipolysis, which has little net effect on HbA1c [15].
A weight loss of 2 to 2.75 is observed in the study and there was a significant decrease of 1.6 in BMI in patients treated with combination therapy of oral hypoglycaemics and Empagliflozin 10 mg (Group-2). An indirect meta-analysis suggests that SGLT-2 inhibitors achieved a greater weight reduction than Dipeptidyl peptidase 4 inhibitors without increasing the risk of hypoglycemia in T2DM patients inadequately controlled with insulin [16]. Weight loss is characterized by early body water and fat loss followed by a slower rate of sustained fat loss. Fat loss is due to glucose loss in the urine with a shift towards lipolysis and ketogenesis [17].
In this study, an approximate reduction of 10 mm of Hg in SBP and 6 mm of Hg reduction in DBP was seen in both groups. Mechanistic trials of short-term and medium-term duration in T2DM showed that SGLT-2 inhibitors produced a modest reduction in 24 h ambulatory BP, and central systolic and pulse pressures [18]. A systolic BP reduction difference was also reported in those allocated to empagliflozin versus placebo treatment in the EMPA-REG OUTCOME trial [19]. There was a reduction in LDL-C, TG and an increase in HDL-C, which is the most vital marker of cardiovascular outcome in T2DM patients. SGLT-2 inhibitors also reduce albuminuria, which reduces kidney damage in patients with Chronic Kidney Disease (CKD). SGLT-2 inhibitors shift the renal threshold for glucose excretion from 180 to 50 mg/dl.
SGLT-2 inhibitors exhibit pleiotropic effects on patients with T2DM, and these include a reduction in BP by decreasing preload and afterload, weight loss by increasing free fatty acid oxidation, hepatic glucose production, and lipolysis, reduced HbA1c by decreasing insulin secretion and increasing glucagon production, and decreases GFR, NHE3, oxidative stress, inflammation, uric acid, producing cardio and reno-protective effects [20]. Meta-analysis trials on SGLT-2 inhibitors yielded better results in the prevention of worsening heart failure and renal disease progression and mortality. Therefore, SGLT-2 inhibitors produce effective cardiovascular and renal outcomes in patients with type 2 DM.
LIMITATIONS
This study was done over a short period and the long-term adverse effects of Empagliflozin on cardiovascular and renal outcomes were not observed, so the results of the study may not apply to long-term effects. The effect on pregnant and lactating women and elderly patients is not proven in this study. Moreover, the study subjects were limited to Osmania General Hospital with relatively better healthcare facilities.
CONCLUSION
A strong reduction in systemic complications is noticed in the treatment of Type 2 DM patients with empagliflozin as an add-on therapy. The study concludes that add-on therapy with empagliflozin illustrates a reduction in HbA1c weight loss, and a reduction in both systolic and diastolic BP, resulting in effective cardiovascular and renal outcomes.
ACKNOWLEDGMENT
We acknowledge Dr. N. Karuna sree, Professor, Department of Pharmacology, Osmania Medical College, Dr. V. Navya, and the staff of the department of pharmacology for their guidance and support.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
Dr. K. VasanthaKumari planned and designed the concept of the manuscript, contributed to drafting the manuscript, and reviewed the manuscript, Dr. Fareen Sulthnasupported in designing and drafting the manuscript, and literature search and review. Dr. Meher Kounen Fatima, Dr. Mohd Moosa QuadriandDr. Chakradhar. Tcontributed to designing the final version to be published.
CONFLICTS OF INTERESTS
Declared none
REFERENCES
World Health Organization. The top 10 causes of death. Available from: http://www.who.int/en/news-room/fact-sheets/detail/the-top-10-causes-of-death. [Last accessed on 02 Feb 2025].
Pradeepa R, Mohan V. Epidemiology of type 2 diabetes in India. Indian J Ophthalmol. 2021;69(11):2932-8. doi: 10.4103/ijo.IJO_1627_21, PMID 34708726.
Anjana RM, Unnikrishnan R, Deepa M, Pradeepa R, Tandon N, Das AK, Joshi S, Bajaj S, Jabbar PK, Das HK, Kumar A, ICMR-INDIAB Collaborative Study Group. Metabolic non-communicable disease health report of India: the ICMR-INDIAB national cross-sectional study (ICMR-INDIAB-17). Lancet Diabetes Endocrinol. 2023 Jul;11(7):474-89.
Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M. Management of hyperglycemia in type 2 diabetes 2015: a patient-centered approach: update to a position statement of the American diabetes association and the European association for the study of diabetes. Diabetes Care. 2015;38(1):140-9. doi: 10.2337/dc14-2441, PMID 25538310.
Ramanathan B, Duraisamy R, Venkatramanasami BT, Abbas MK, Balamurugan A. Association of glycaemic status and outcomes in diabetic foot problems: a retrospective evidence from South India. J Basic Clin Physiol Pharmacol. 2021;33(2):155-62. doi: 10.1515/jbcpp-2020-0198, PMID 33618439.
Ismail L, Materwala H, Al Kaabi J. Association of risk factors with type 2 diabetes: a systematic review. Comput Struct Biotechnol J. 2021 Mar 10;19:1759-85. doi: 10.1016/j.csbj.2021.03.003, PMID 33897980.
Bhatti JS, Sehrawat A, Mishra J, Sidhu IS, Navik U, Khullar N. Oxidative stress in the pathophysiology of type 2 diabetes and related complications: current therapeutics strategies and future perspectives. Free Radic Biol Med. 2022 May 1;184:114-34. doi: 10.1016/j.freeradbiomed.2022.03.019, PMID 35398495.
Batista TM, Haider N, Kahn CR. Defining the underlying defect in insulin action in type 2 diabetes. Diabetologia. 2021;64(5):994-1006. doi: 10.1007/s00125-021-05415-5, PMID 33730188.
Lunati ME, Cimino V, Gandolfi A, Trevisan M, Montefusco L, Pastore I. SGLT2-inhibitors are effective and safe in the elderly: the sold study. Pharmacol Res. 2022 Sep;183:106396. doi: 10.1016/j.phrs.2022.106396, PMID 35970329.
Triplitt CL, Reasner CA, Isley WL. Diabetes mellitus. In: Di Piro JT, Talbert RL, Yee GC, editors. Pharmacotherapy: a pathophysiologic approach. 7th ed. New York: McGraw-Hill; 2008. p. 1205-41.
Frampton JE. Empagliflozin: a review in type 2 diabetes. Drugs. 2018 Jul;78(10):1037-48. doi: 10.1007/s40265-018-0937-z, PMID 29946963.
Paczkowska A, Hoffmann K, Michalak M, Bryl W, Kopciuch D, Zaprutko T. A comparison between the therapeutic effect of metformin alone versus combination therapy with insulin in uncontrolled non-adherence patients with type 2 diabetes: six months follow up. Diabetes Metab Syndr Obes. 2021 Jul 14;14:3243-52. doi: 10.2147/DMSO.S317659, PMID 34285531.
Anker SD, Usman MS, Butler J. SGLT2 inhibitors: from antihyperglycemic agents to all around heart failure therapy. Circulation. 2022 Jul 26;146(4):299-302. doi: 10.1161/circulationaha.122.060348, PMID 35877834.
Ndefo UA, Anidiobi NO, Basheer E, Eaton AT. Empagliflozin (Jardiance): a novel SGLT2 inhibitor for the treatment of type 2 diabetes. PT. 2015 Jun;40(6):364-8, PMID 26045645.
Inzucchi SE, Davies MJ, Khunti K, Trivedi P, George JT, Zwiener I. Empagliflozin treatment effects across categories of baseline HbA1c body weight and blood pressure as an add on to metformin in patients with type 2 diabetes. Diabetes Obes Metab. 2021 Feb;23(2):425-33. doi: 10.1111/dom.14234, PMID 33084149.
Vallon V, Verma S. Effects of SGLT2 inhibitors on kidney and cardiovascular function. Annu Rev Physiol. 2021 Feb 10;83:503-28. doi: 10.1146/annurev-physiol-031620-095920, PMID 33197224.
Min SH, Yoon JH, Hahn S, Cho YM. Comparison between SGLT2 inhibitors and DPP4 inhibitors added to insulin therapy in type 2 diabetes: a systematic review with indirect comparison meta-analysis. Diabetes Metab Res Rev. 2017;33(1):e2818. doi: 10.1002/dmrr.2818, PMID 27155214.
Chacko J, Dhandapani S, Jahagiridhar V, Swaminathan K. The effects of empagliflozin on cardiometabolic risk factors in patients with type 2 diabetes: a short-term observational study. Indian J Pharmacol. 2021 May-Jun;53(3):229-33. doi: 10.4103/ijp.IJP_669_18, PMID 34169909.
Baker WL, Smyth LR, Riche DM, Bourret EM, Chamberlin KW, White WB. Effects of sodium-glucose co-transporter 2 inhibitors on blood pressure: a systematic review and meta-analysis. J Am Soc Hypertens. 2014;8(4):262-75.e9. doi: 10.1016/j.jash.2014.01.007, PMID 24602971.
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S. Empagliflozin cardiovascular outcomes and mortality in type 2 diabetes. N Engl J Med. 2015 Nov 26;373(22):2117-28. doi: 10.1056/NEJMoa1504720, PMID 26378978.
Takata T, Isomoto H. Pleiotropic effects of sodium-glucose cotransporter 2 inhibitors: renoprotective mechanisms beyond glycemic control. Int J Mol Sci. 2021 Apr 22;22(9):4374. doi: 10.3390/ijms22094374, PMID 33922132.