
Int J Pharm Pharm Sci, Vol 17, Issue 3, 16-20Original Article
A CROSS-SECTIONAL OBSERVATIONAL STUDY TO EVALUATE THE EFFICACY OF METHYLENE BLUE IN THE TREATMENT OF HYPOXIC COVID-19 PATIENTS
NARENDER SAYINI1*, PADMA RACHARLA2
1Department of Pulmonology GGH, Karimnagar, Telangana-505001, India. 2Department of General Medicine, GGH, Karimnagar, Telangana-505001, India
*Corresponding author: Narender Sayini; *Email: sayininarender0@gmail.com
Received: 20 Nov 2024, Revised and Accepted: 05 Feb 2025
ABSTRACT
Objective: The primary objective of this study is to evaluate the effectiveness of Methylene Blue (MB) in reducing hypoxia in COVID-19 patients.
Methods: A retrospective cross-sectional observational record-based study was conducted at Sai Krishna Multi-Specialty Hospital, Karimnagar, Telangana, over four months with 100 case sheets. A paired equal variance T-test was used to compare the pre-and post-treatment levels of SpO2 and respiratory rate among the hypoxic patients.
Results: Out of 100 case sheets examined, about 70 patients were between the age groups of 45 to 54 years, 58 were males and 42 were females. The mean SPO2 before treatment was 71.47% compared with day 3 values of SPO2, which is 77.76%, and there was a significant improvement in the mean saturation levels with a P value less than 0.0001. There was a significant improvement in the mean respiratory rate, which was 26.92+0.85 at baseline before treatment, 23.93+0.90 on Day 3, and 21.42+1.27 on Day 7 after treatment with methylene blue and the P value is less than 0.0001.
Conclusion: This study concludes that Methylene blue intervention in COVID-related acute respiratory distress syndrome avoids the devastating consequences and improves patients’ condition by reducing hypoxemia and respiratory distress.
Keywords: Acute respiratory distress syndrome, Hypoxemia, Respiratory rate
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijpps.2025v17i3.53238 Journal homepage: https://innovareacademics.in/journals/index.php/ijpps
INTRODUCTION
Corona Virus is an RNA virus that causes severe acute respiratory distress syndrome characterized by multi-organ failure, respiratory distress, and excessive inflammation, including cytokine storm, brought on by the virus spreading through the blood [1, 2]. The strain of coronavirus responsible for the COVID-19 pandemic is Severe Acute Respiratory Syndrome Coronavirus 2(SARS-CoV-2). 6.6 million people worldwide have died from this dreadful Covid-19 sickness as of November 2022 [3]. The cascade of events in the pathogenesis of SARS-CoV-2 infection is viral invasion and replication, a dysregulated immune response, multiple organ damage, and recovery [4]. The anti-viral drugs, immunotherapy, and cell therapy were effective only if they were administered before the initiation of the inflammatory cascade. Several drugs like chloroquine, hydroxychloroquine, azithromycin, lopinavir, ritonavir, ribavirin, tocilizumab, baricitinib, remdesivir, favipiravir, ruxolitinib, teicoplanin, ivermectin, doxycycline, corticosteroids, heparin and NSAIDS were used [5]. Several vaccines were also discovered to combat COVID-19 and these include Covishield, Covaxin, Sputnik V, Pfizer BioNtech, Moderna, Zydus, etc [6].
Despite the administration of a high percentage of Oxygen support by non-invasive and invasive ventilation in COVID patients, there was continued hypoxemia and increased respiratory distress [7]. This paved the way for the introduction of Methylene blue in the treatment of COVID-19 patients with disproportional hypoxemia and distress. Methylene blue (MB) has indeed been explored as a potential treatment for COVID-19, particularly for patients suffering from hypoxemia (low oxygen levels in the blood) and respiratory distress, including those who experience severe lung damage or acute respiratory distress syndrome (ARDS). Methylene blue is a phenothiazine dye that has a variety of medical applications due to its ability to act as a redox agent (helping in electron transfer), and its effects on mitochondrial function. Methylene blue occurs in two forms, which are oxidised and reduced forms. The reduced form is an anti-oxidant and alleviates oxidative stress, whereas the oxidized form is an oxidant and elevates oxidative stress. Therefore, reduced methylene blue also known as Leucomethylene blue, is used for treating severely hospitalized COVID-19 patients [8]. Other established medical uses of Methylene Blue are antidote for cyanide poisoning, photodynamic therapy, Hepatitis C and HIV, hypotension in sepsis and vasoplegia, psoriasis, and diagnostic marker [8, 9].
The various effects of Methylene blue against the SARS-C0V-2 virus are as follows [10-12]:
Methylene blue has been found to improve mitochondrial function and enhance cellular oxygen utilization. MB helps by improving the efficiency of oxygen usage at the cellular level. It can act on the cytochrome c oxidase in the electron transport chain of mitochondria, potentially improving oxygen consumption and reducing hypoxic conditions. This could theoretically assist in reversing the oxygen deficit in tissues affected by COVID-19.
Methylene blue has antioxidant properties, meaning it can neutralize reactive oxygen species (ROS) and reactive nitrogen species (RNS), which are produced in excess during infections like COVID-19, contributing to tissue damage and inflammation. By mitigating oxidative stress, MB might help reduce the inflammation that contributes to lung injury and other systemic effects of the virus. This could potentially help patients experiencing cytokine storms, a key feature of severe COVID-19, where excessive inflammation causes widespread tissue damage. Inhibits binding of spike protein to host receptor, impairs membrane fusion resulting in reduced viral entry. Reduces viral uncoating and protein translation by increasing lysosomal pH, and inhibits RNA-dependent RNA polymerase through zinc ionophores, resulting in reduced viral replication. Inhibits NLRP3, and reduces excess nitric oxide activity and reactive oxygen species activity, resulting in reduced hyperinflammation.
MB has been used to treat hypotension (low blood pressure), particularly in conditions like sepsis or vasoplegia (a state of extreme blood vessel dilation). In severe COVID-19 cases where blood pressure drops and organ perfusion is compromised, MB may help improve hemodynamic stability and restore proper blood circulation.
The study aimed to determine the effectiveness of Methylene Blue in hypoxic COVID-19 patients by comparing the peripheral capillary oxygen saturation (SpO2) and respiratory rate at baseline with those values after treatment with Methylene Blue.
MATERIALS AND METHODS
The study was a retrospective cross-sectional observational record-based study. Case sheets of patients diagnosed with COVID-19 and hospitalized due to severe hypoxemia and respiratory distress were used to perform the analysis. It was conducted at Sai Krishna multi-specialty hospital, Karimnagar, Telangana, and lasted four months, from May 2021 to August 2021. The consent to conduct the study was taken from the head of the Department of Pulmonology, Sai Krishna Hospital, Karimnagar, Telangana. SpO2 and respiratory rate were analyzed based on convenience sampling using Case sheets of COVID-19 patients treated with methylene blue to reduce hypoxemia and respiratory distress.
Peripheral capillary oxygen saturation (SpO2) is commonly measured by pulse oximetry, which provides an indirect measurement of arterial oxygenation (SaO2) based on the differential absorption of light by oxygenated and deoxygenated blood during pulsatile blood flow [13]. Normal oxygen saturation at sea level ranges between 96% and 100% [14]. In clinical settings, respiratory rate is frequently used as a screening tool to detect respiratory tract infections. According to standards, tachypnea is a respiratory rate (RR) of more than 20 respirations per minute (rpm) [15]. The severity of respiratory distress was assessed clinically based on hypoxemia (SPO2) and tachypnoea (RR).
Study subjects
Case sheets of 100 patients diagnosed with COVID-19 and with a history of severe hypoxemia (SPO2 of 70%) and respiratory distress admitted at Sai Krishna multi-specialty hospital were evaluated to assess the effect of methylene blue in the treatment of COVID-19 patients.
Inclusion criteria
Case sheets of men and women aged 18 years and above diagnosed with COVID-19 through RTPCR and hospitalized due to severe hypoxemia and respiratory distress with baseline characteristics of acute onset, diffuse infiltrations, SPO2<75% and RR>26 rpm were included in this study.
Exclusion criteria
Case sheets of pregnant and lactating women, prior administration of methylene blue 30 days before hospitalization, patients with known hypersensitivity to methylene blue, those on other standard treatment, and patients whose death is inevitable within 24 h of admission based on clinical assessment.
Methods
A retrospective cross-sectional observational study was conducted. A total of 100 case sheets were included in the study. Those patients were treated with 100 mg of Methylene blue (SAM-MB) in 500 ml NS through IV route thrice daily. No other standard treatment was given. Baseline parameters such as SPO2 and respiratory rate were studied and compared with the values on the third and seventh day of admission. The collected data was tabulated and analyzed using MS Excel and SPSS software version 26.
Statistical analysis
The data collected was entered in Microsoft Excel and the analysis was performed using the software package for the social sciences (SPSS) version 26. The level of significance was taken as 0.05; therefore, the p-values of<0.05 were considered significant. Initially, the baseline characteristics were analyzed and then compared with post-treatment parameters to evaluate the treatment effects. Paired Equal Variance Two-tailed T-test was used for the analysis of continuous variables.
Primary outcome measures
Recovery from hypoxemia and tachypnoea based on pre-and post-treatmentSPO2, RR. Recovery from hypoxemia is defined as the duration of air-breathing after an acute hypoxic exposure that raised and normalized peripheral blood SpO2 to>96%. Recovery from tachypnoea is defined as the reduction in respiratory rate to a normal range of 12-20 breaths per minute.
RESULTS
Secondary outcome measures
Clinical response and overall therapeutic response based on general cure or treatment failure. Clinical response is the reduction in clinical symptoms (hypoxemia and tachypnoea) and overall therapeutic response is the total improvement in response to treatment with methylene blue.
Table 1: Age-wise distribution of patients
| Age group in years | No. of patients | Mean+SD |
| 35-39 | 12 | 37.08+1.62 |
| 40-44 | 8 | 43.37+1.40 |
| 45-49 | 33 | 46.87+1.38 |
| 50-54 | 37 | 52.05+1.41 |
| 55-59 | 10 | 57+1.49 |
Using Microsoft Excel and SPSS software, the mean age and standard deviation of the patients were calculated.
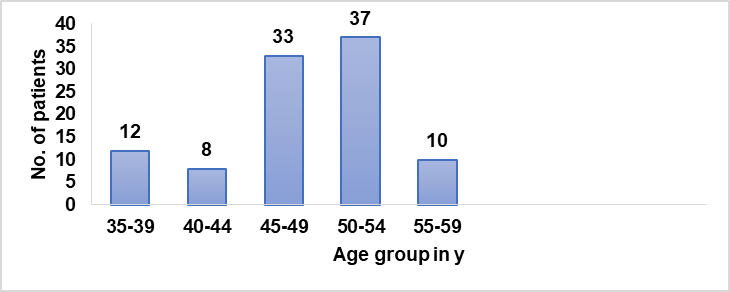
Graph 1: Age-wise distribution of patients
Table 2: Gender-wise distribution of patients
| Gender | No. of patients | Percentage |
| Males | 58 | 58% |
| Females | 42 | 42% |
| Total | 100 | 100% |
Using Microsoft Excel, the percentage of male and female patients was calculated.

Graph 2: Gender-wise distribution of patients
Table 3: Comparison of baseline, Day 3 and Day 7 SPO2
| Parameter | Mean+SD | P value |
| Baseline SPO2 | 71.47+1.22 | *P<0.00001 |
| Day 3 SPO2 | 77.76+1.80 | |
| Day 7 SPO2 | 89.92+1.43 |
*Paired equal variance two-tailed t-test showing a significant improvement in post-treatment SPO2 levels.
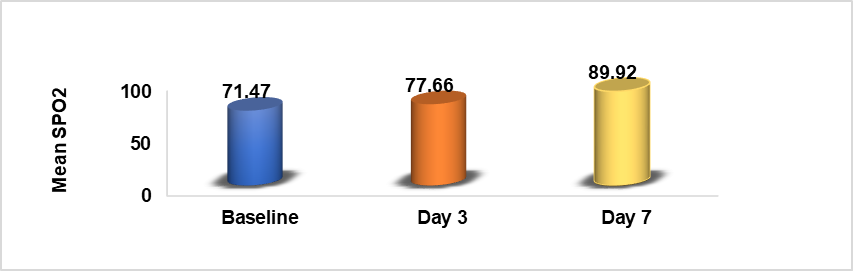
Graph 3: Comparison of baseline and day 3 and day 7 SPO2
Table 4: Comparison of baseline, Day 3, and day 7RR
| Parameter | Mean+SD | P value |
| Baseline RR | 26.92+0.85 | P<0.00001 |
| Day 3 RR | 23.93+0.90 | |
| Day 7 RR | 21.42+1.27 |
*Paired Equal Variance Two-tailed T-test showing a significant improvement in post-treatment reduction of respiratory rate.
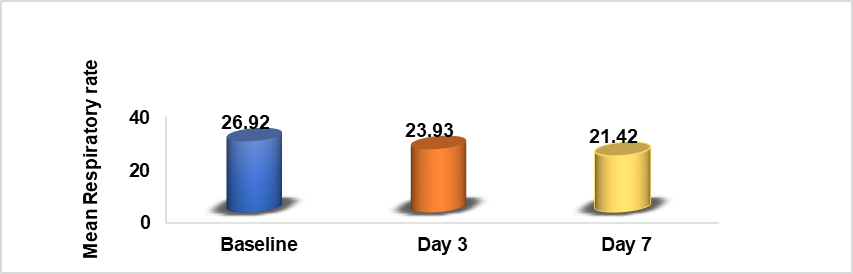
Graph 4: Comparison of baseline and day 3 and day 7 RR
Demographic factors
Age distribution: The study found that patients between the ages of 45 and 49 were most affected by COVID-19, as indicated in table 1 and Graph 1.
Gender distribution: Males were found to be more affected than females, as shown in table 2 and Graph 2. Specifically, males aged 45-54 seemed to be the most impacted group, suggesting a gender and age-based vulnerability to COVID-19.
Clinical observations and methylene blue treatment
Oxygen saturation (SpO2) levels: The mean SpO2 before treatment was compared with values on Day 3 and Day 7. A significant improvement in oxygen saturation was noted, with P<0.05, indicating that Methylene blue significantly improved hypoxemia. Table 3 and Graph 3 show the progression of mean SpO2 levels from baseline to Day 3 and Day 7, demonstrating the positive effects of Methylene blue on oxygenation in COVID-19 patients.
Respiratory rates: Respiratory rates were recorded at baseline, Day 3, and Day 7. A significant reduction in tachypnea (rapid breathing) was observed, with P<0.0001 indicating a strong correlation between MB treatment and improved respiratory function. The findings in table 4 and Graph 4 highlight that Methylene blue helped reduce abnormal breathing patterns, a common complication in severe COVID-19 cases.
Symptom relief and recovery: After treatment with Methylene blue, there was a notable reduction in several symptoms such as fatigue, dry cough, headache, fever, and loss of smell, which are frequently observed in COVID-19 patients. The duration of hospital stay was also reduced, indicating a faster recovery with Methylene blue treatment. All patients treated with Methylene blue fully recovered within 1 to 2 mo, suggesting its effectiveness in promoting recovery and improving the overall health condition of COVID-19 patients.
Methylene blue's effectiveness: Treatment with Methylene blue demonstrated:
Significant improvement in oxygen saturation levels and respiratory rates, with results showing statistical significance (P<0.05 and P<0.0001). Reduction in symptoms like fatigue, cough, fever, and loss of smell. Shorter hospital stays and complete recovery in 1-2 mo. This suggests that Methylene blue has therapeutic potential in mitigating hypoxemia, reducing tachypnea, and improving recovery times in COVID-19 patients, making it a valuable adjunct to traditional COVID-19 treatments. However, these findings would benefit from further clinical validation and larger-scale studies to establish the full scope of its effectiveness in a broader patient population.
DISCUSSION
Methylene blue is a tricyclic phenothiazine dye used to treat COVID-19 patients with severe hypoxemia and respiratory distress [11]. It was evident that the lungs were the most affected organs in patients with COVID-19 due to the invasion of the virus and damage to the respiratory endothelium resulting in inflammation leading to hypoxemia and respiratory distress [16]. Studies have shown that the addition of Methylene blue in the COVID-19 treatment protocol enhanced the oxygen saturation levels and reduced respiratory distress, shortened hospital stay, and reduced mortality of patients [17]. Therefore, in the present study, the efficacy of methylene blue in hypoxic COVID-19 patients was evaluated.
In this study, the common age group of affected patients ranged from 44 to 55 years and the median age was 49 years, consistent with a similar study conducted by Chaolin Huang et al. [18]. Males were more affected than females and the results were consistent with a similar study conducted by Meng Y et al. [19]. This might be due to higher expression of ACE-2, lifestyle behavior such as smoking and drinking, and immunological differences driven by sex hormones and X chromosomes [20]. There was a significant improvement in SPO2 and respiratory rate after treatment of patients with methylene blue. A similar study conducted by D. Hamidi-Alamdari et al. concluded that an addition of MB to the treatment protocols significantly improved SpO2 and respiratory distress in COVID-19 patients, which resulted in decreased hospital stay and mortality [17]. Sanei ZS et al. conducted a study and observed that the oral administration of Methylene blue demonstrated positive effects on improving SpO2 levels and reducing inflammatory markers in COVID-19-related ARDS patients [21]. Methylene blue is also effective in the treatment of bovine Coronavirus in vitro [22]. Methylene blue served as a life-saving drug in the treatment of hypoxemia and respiratory distress in hospitalized COVID-19 patients [23].
LIMITATIONS OF THE STUDY
The results of the study cannot be generalized as the study was a cross-sectional observational study conducted in a single hospital. The duration of the study is also small and conducted over a small group of patients. The efficacy would have been better evaluated if it was a large multi-centric study conducted among a large group of patients for a longer duration.
CONCLUSION
In hospitalized patients with COVID-19, the major symptoms were hypoxemia and respiratory distress. As these symptoms may aggravate and result in the death of the patient, it is important to choose a drug that improves oxygen saturation and reduces respiratory distress. In this hospital Inj. Methylene blue was used to combat the ARDS symptoms. This study conducted using Case sheets of treated patients concludes that Methylene blue intervention in COVID-related ARDS avoids the devastating consequences and improves patients’ condition.
ACKNOWLEDGMENT
We acknowledge the staff of Sai Krishna multi-specialty Hospital for their constant support and assistance.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
Dr. Narender Sayini has planned, designed the concept of the manuscript, and drafted the manuscript, Dr. Padma Racharla has supported in drafting and collecting the reference articles.
CONFLICTS OF INTERESTS
Declared none
REFERENCES
Rani AS, Madhavi P, Chakradhar T. A retrospective study to evaluate the efficacy of injection augmentin in COVID-19 patients with pneumonia at a Tertiary Care Teaching Hospital Telangana. Int J Pharm Pharm Sci. 2022;14(10):28-31. doi: 10.22159/ijpps.2022v14i10.45730.
Scigliano G, Scigliano GA. Methylene blue in COVID-19. Med Hypotheses. 2021;146:110455. doi: 10.1016/j.mehy.2020.110455, PMID 33341032.
Swarupa K, Sravani M, Karunasree N. Assessment and analysis of adverse events following COVID-19 vaccination among children aged 15-18 y at a Tertiary Care Teaching Hospital Telangana: a prospective observational study. Int J Pharm Pharm Sci. 2023;15(3):15-20. doi: 10.22159/ijpps.2023v15i3.47358.
Bohn MK, Hall A, Sepiashvili L, Jung B, Steele S, Adeli K. Pathophysiology of COVID-19: mechanisms underlying disease severity and progression. Physiology (Bethesda). 2020;35(5):288-301. doi: 10.1152/physiol.00019.2020, PMID 32783610.
Mirtaleb MS, Mirtaleb AH, Nosrati H, Heshmatnia J, Falak R, Zolfaghari Emameh RZ. Potential therapeutic agents to COVID-19: an update review on antiviral therapy immunotherapy and cell therapy. Biomed Pharmacother. 2021;138:111518. doi: 10.1016/j.biopha.2021.111518, PMID 33774315.
Sabitha S, Shobana N, Prakash P, Padmanaban S, Sathiyashree M, Saigeetha S. A review of different vaccines and strategies to combat COVID-19. Vaccines (Basel). 2022 May 9;10(5):737. doi: 10.3390/vaccines10050737, PMID 35632493, PMCID PMC9145217.
Shenoy N, Luchtel R, Gulani P. Considerations for target oxygen saturation in COVID-19 patients: are we undershooting? BMC Med. 2020 Aug 19;18(1):260. doi: 10.1186/s12916-020-01735-2, PMID 32814566.
Dabholkar N, Gorantla S, Dubey SK, Alexander A, Taliyan R, Singhvi G. Repurposing methylene blue in the management of COVID-19: mechanistic aspects and clinical investigations. Biomed Pharmacother. 2021 Oct;142:112023. doi: 10.1016/j.biopha.2021.112023, PMID 34399199.
OZ M, Lorke DE, Hasan M, Petroianu GA. Cellular and molecular actions of methylene blue in the nervous system. Med Res Rev. 2011 Jan;31(1):93-117. doi: 10.1002/med.20177, PMID 19760660.
Hepburn J, Williams Lockhart S, Bensadoun RJ, Hanna R. A novel approach of combining methylene blue photodynamic inactivation photobiomodulation and oral ingested methylene blue in COVID-19 management: a pilot clinical study with 12-month follow-up. Antioxidants (Basel). 2022 Nov 8;11(11):2211. doi: 10.3390/antiox11112211, PMID 36358582.
Chuang ST, Papp H, Kuczmog A, Eells R, Condor Capcha JM, Shehadeh LA. Methylene blue is a nonspecific protein-protein interaction inhibitor with potential for repurposing as an antiviral for COVID-19. Pharmaceuticals (Basel). 2022 May 18;15(5):621. doi: 10.3390/ph15050621, PMID 35631447.
Cagno V, Medaglia C, Cerny A, Cerny T, Zwygart AC, Cerny E. Methylene blue has a potent antiviral activity against SARS-CoV-2 and H1N1 influenza virus in the absence of UV-activation in vitro. Sci Rep. 2021 Jul 12;11(1):14295. doi: 10.1038/s41598-021-92481-9, PMID 34253743.
Ascha M, Bhattacharyya A, Ramos JA, Tonelli AR. Pulse oximetry and arterial oxygen saturation during cardiopulmonary exercise testing. Med Sci Sports Exerc. 2018 Oct;50(10):1992-7. doi: 10.1249/MSS.0000000000001658, PMID 29771822.
Torp KD, Modi P, Pollard EJ. Pulse oximetry. In: Treasure Island, (FL): Stat Pearls Publishing; 2023 Jul 30. p. 2024.
Miller DJ, Capodilupo JV, Lastella M, Sargent C, Roach GD, Lee VH. Analyzing changes in respiratory rate to predict the risk of COVID-19 infection. Plos One. 2020 Dec 10;15(12):e0243693. doi: 10.1371/journal.pone.0243693, PMID 33301493.
Polidoro RB, Hagan RS, DE Santis Santiago R, Schmidt NW. Overview: systemic inflammatory response derived from lung injury caused by SARS-CoV-2 infection explains severe outcomes in COVID-19. Front Immunol. 2020;11:1626. doi: 10.3389/fimmu.2020.01626, PMID 32714336.
Hamidi Alamdari D, Hafizi Lotfabadi S, Bagheri Moghaddam A, Safari H, Mozdourian M, Javidarabshahi Z. Methylene blue for treatment of hospitalized COVID-19 patients: a randomized controlled open-label clinical trial phase 2. Rev Invest Clin. 2021;73(3):190-8. doi: 10.24875/RIC.21000028, PMID 34019535.
Huang C, Wang Y, LI X, Ren L, Zhao J, HU Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan China. Lancet. 2020;395(10223):497-506. doi: 10.1016/S0140-6736(20)30183-5, PMID 31986264.
Meng Y, WU P, LU W, Liu K, MA K, Huang L. Sex-specific clinical characteristics and prognosis of coronavirus disease-19 infection in Wuhan China: a retrospective study of 168 severe patients. Plos Pathog. 2020 Apr 28;16(4):e1008520. doi: 10.1371/journal.ppat.1008520, PMID 32343745.
Bwire GM. Coronavirus: why men are more vulnerable to COVID-19 than Women? SN Compr Clin Med. 2020;2(7):874-6. doi: 10.1007/s42399-020-00341-w, PMID 32838138.
Sanei ZS, Shahrahmani F, Khaleghi Manesh B, Hamidi Alamdari D, Mehrad Majd H, Mavaji Darban B. Methylene blue for COVID-19 ARDS: insights from a randomized clinical trial. Naunyn Schmiedebergs Arch Pharmacol. 2024 Aug 29. doi: 10.1007/s00210-024-03371-6, PMID 39207597.
Zhukhovitsky V, Shevlyagina N, Zubasheva M, Russu L, Gushchin V, Meerovich G. Infectivity and morphology of bovine coronavirus inactivated in vitro by cationic photosensitizers. Viruses. 2022 May 15;14(5):1053. doi: 10.3390/v14051053, PMID 35632792.
Yella SH, Yella SS, Sasanka KS, Thangaraju P. Does methylene blue satisfy an option in COVID-19 ARDS? Infect Disord Drug Targets. 2022;22(6):e170322202339. doi: 10.2174/1871526522666220317155947, PMID 35301956.