
Int J Pharm Pharm Sci, Vol 17, Issue 7, 9-20Review Article
A COMPREHENSIVE REVIEW ON MICROSPONGE
SATYAJIT SAHOO1*, SOHAN PATEL, SAPNA DESAI, TEJAS PATEL
Pioneer Pharmacy Degree College, Vadodara, Gujarat, India
*Corresponding author: Satyajit Sahoo; *Email: satyajitppdc@gmail.com
Received: 07 Apr 2025, Revised and Accepted: 28 May 2025
ABSTRACT
The objective of the present study was to gain knowledge and update ourselves about the Micro sponges and its applications in Pharmacy. It is a unique delivery system. It comprises of porous micro spheres ranging in size from 5 to 300 microns. It comprises of porous micro spheres ranging in size from 5 to 300 microns. This small size easily penetrate the pores or channels of skin. It can be used to produce controlled release formulations of poorly soluble drugs. It increases drug stability, reduces side effects and modifies drug release profiles making it a versatile drug delivery vehicle. These active micro sponges can entrap a wide variety of substances. It can be incorporated into formulations, such as capsules, gels, liquids, creams and powders. Thus, it can be used for the controlled release of topical agents. Also, it can be used for oral, ophthalmic and parenteral route. Now-a-days it is widely applied in the field of cosmetic and dermatology. This review article covers methods of preparation, release mechanism, assessment parameters, applications and marketed product of micro sponges delivery system.
Keywords: Micro sponge, Porous micro sphere, Poor soluble drugs, Controlled release, Topical preparation, Cosmetic, Dermatology
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijpps.2025v17i7.54500 Journal homepage: https://innovareacademics.in/journals/index.php/ijpps
INTRODUCTION
Now-a-days the major challenge faced by cosmetic industry is control the release rate of drug in cosmetic formulation. Drug delivery technology has become highly competitive and rapidly evolving [1]. Therefore, researcher focused on developing some unique controlled release drug delivery system to improve its efficacy, cost effectiveness to increase patient compliance. Some challenges faced by formulation developing industries are have controlled release technology for reducing skin irritation and enhance patient compliance to give long term product efficacy [2].
In the past years, development of only drug is not sufficient it also requires a suitable drug release system which can control the release rate of drug. For this challenge’s carrier technology is the best solution [3]. This includes micro sphere, nano particle, liposomes etc., which alter the absorption and release characteristics of the drug.
Micro spheres [4] are unable to control the release rate of the drug from itself. Once the outer wall is ruptured the drug contained within micro spheres will be released from it. Liposomes [5] having demerits like lower drug entrapment, difficulty in preparing formulation, limited chemical stability and microbial stability. The Micro sponges based systems can overcome the problems associated with above carrier systems. Micro sponge has been colossal innovation in pharmaceutical field [6]. A Micro sponge Delivery System is patented, highly cross-linked, porous, polymeric micro spheres that can entrap wide range of actives and then release them with desired rate. This system is useful for the improvement of performance of topically applied drugs. It is a unique technology for the controlled release of topical agents and consists of micro porous beads, loaded with active agent. Their high degree of cross-linking results in particles that are insoluble, inert and of sufficient strength to stand up to the high shear commonly used in manufacturing of creams, gels, lotions, and powders. Their characteristic feature is the capacity to adsorb or “load” a high degree of active materials into the particle and on to its surface [7, 8].
 |
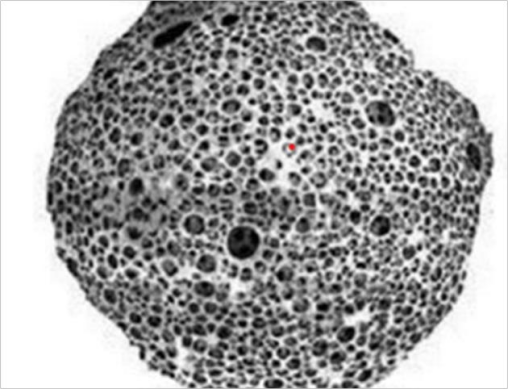 |
| Fig. 1(a): Microsponge [4, 7] | Fig. 1(b): Porous structure of microsponge [4, 7] |
History of micro sponge
The micro sponge technology was developed by Won in 1987, and the original patents were assigned to Advanced Polymer System, Inc [9]. This company developed a large number of variations of the technique and applied those to the cosmetic as well as over-the-counter (OTC) and prescription pharmaceutical products. At the present time, this interesting technology has been licensed to Cardinal Health, Inc., for use in topical products. The size of microsponge can be varied, usually from 5-300 µm in diameter, depending upon degree of smoothness or afterfeel required for the end formula [10].
Structure of microsponge
The size of the micro sponge [11] is usually ranges from 5–300 μm in diameter, depending upon the degree of smoothness. The micro sponge sphere can have up to 250000 pores and an internal pore structure equivalent to 10 ft in length, providing a total pore volume of about 1 ml/g. This shows a large reservoir within each.
Micro sponge, which can be loaded with up to its own weight of active agent. The micro sponge particles itself are too large to be absorbed into the skin and this can adds a measure of safety to these micro sponge materials. As the size of the pore diameter is smaller, the bacteria ranging from 0.007 to 0.2 μm cannot penetrate into the tunnel structure of the micro sponges [12]. Release of active ingredients from conventional topical formulation over an extended period of time is quite difficult. In contrast, micro sponges technology allow an even and sustained rate of release of active ingredient, reducing irritation while maintaining efficacy. They have high degree of cross-linking result in particles that are inert of potential strength, insoluble to stand up to the high shear mostly used in manufacturing of creams, lotion, and powder [13].
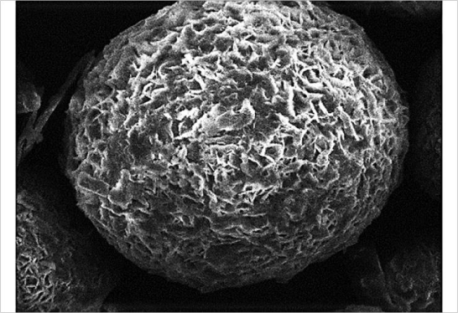
Fig. 2: Structure of microsponge [4]
Characteristics of micro sponges
These are stable over range of pH 1 to 11 [14]. These are stable at the temperature up to 130 °C. These are compatible with most vehicles and ingredients [15]. These are self-sterilizing as their average pore size is 0.25µm where bacteria cannot penetrate [16]. They have higher payload (50 to 60%), still free flowing and can be cost effective [17].
Characteristics of active ingredients entrapped into micro sponges
It should be either miscible in monomer as well as capable of being made miscible by addition of small amount of a water immiscible solvent [18]. It should be inert to monomers and should not increase the viscosity of the mixture during formulation.
It should be water immiscible or nearly only slightly soluble [19]. It should not collapse spherical structure of the micro sponge. It should be stable in contact with polymerization catalyst and also in conditions of polymerization. Not more than 10 to 12% w/w micro sponge must be incorporated in to the vehicle in order to avoid cosmetic problems [20]. Payload and polymer design of the micro sponge for active must be optimized for required release rate for given period of time. Use of organic solvents as porogens, pose an environmental hazard which may be highly inflammable.
Mechanism of drug release from microsponge
The active ingredient is added to the vehicle in an entrapped form. As the micro sponge particle has an open structure as there is no continuous membrane surrounding them and the active ingredient moves freely in and out from the micro sponge particles and into the vehicle until equilibrium is reached, when the vehicle becomes saturated [24]. Once the finished product is applied on the skin, the active agent is already present in the vehicle will be absorbed into the skin, depleting the vehicle, which will become unsaturated, therefore, disturbing the equilibrium. This will begin a flow of the micro sponge particle into the vehicle, and then it to the skin, until the vehicle is either absorbed or dried [25].
Even after that the micro sponge particles continue to have on the stratum corneum surface and continue to gradually release the active agent to the skin and providing prolonged release over time [26].
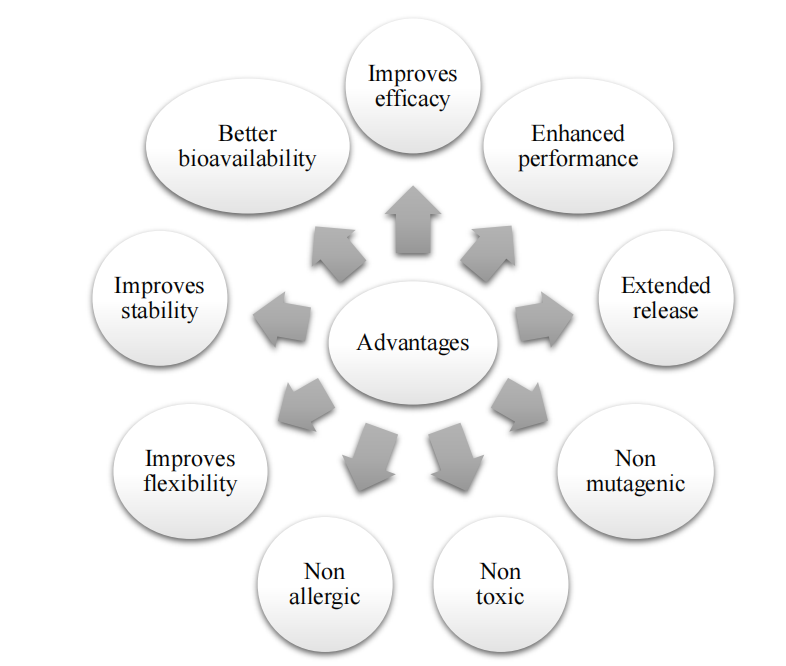
Fig. 3: Advantages of micro sponges [21]
Table 1: Advantages of micro sponges over
| Conventional formulations | Micro encapsulation and liposomes |
| Conventional formulations of topical drugs are intended to work on the outer layers of the skin. Such products release their active ingredients upon application, producing a highly concentrated layer of active ingredient that is rapidly absorbed. When compared to the conventional system, Micro sponge systems can prevent excessive accumulation of ingredient within the epidermis and the dermis significantly reducing the irritation of effective drugs without affecting their efficacy [22]. | Micro capsules cannot usually control the release rate of actives. Once the wall is ruptured, the actives contained within micro capsules will be released. Liposomes suffer from lower payload, difficulty in formulation, limited chemical and microbial stability, whereas micro sponge system in contrast to the above system has several advantages like stable over a pH range of 1-11 and up to temperature of 130 ⁰C, have higher payload up to 50 to 60 %, with average pore size of 0.25 μm where bacteria cannot penetrate. MSP systems maximize the amount of time that an active ingredient is present either on skin surface or within the epidermis, while minimizing its transdermal penetration into the body [23]. |
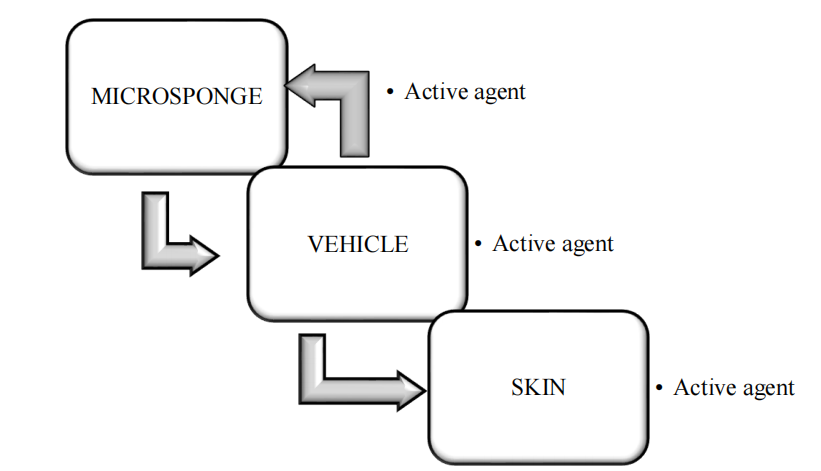
Fig. 4(a): Drug release from microsponge [25]
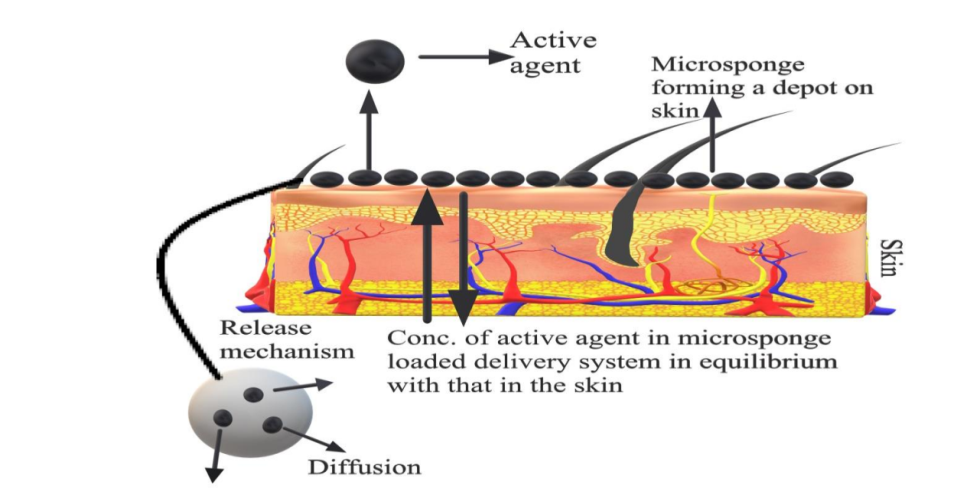
Fig. 4(b): Mechanism of drug release from topical microsponges [28]
During the compounding of finished products if the active is very soluble in the preferred vehicle, the products will not offer the preferred benefits of gradual release. Instead, they will behave as if the active was added to the vehicle in a free form [27]. Therefore, while formulating micro sponge entrapment's, it is significant to design a vehicle that has low solubility power for the actives [28]. This principle is opposite to the conventional formulation principles generally applied to the topical products. For these conventional systems it is usually recommended to maximize the solubility of the active in the vehicle [29]. When using micro sponge entrapment, a few of the active in the vehicle is acceptable, because the vehicle can give the initial loading dose of the active until release from the micro sponge is activated by the shift in equilibrium into the carrier from the polymer [30]. Another method to avoid undesirable premature leaching of active from the micro sponge polymer is to formulate the product with some entrapped active and some free, so the vehicle is pre saturated. In this case there will not be any leaching of the active from the polymer during compounding process [31]. The rate of active release will finally not depend on the partition coefficient of the active ingredient between the polymer and the vehicle (or the skin), but also on a few of the parameters that characterize the bead. Example for these includes surface area and primarily, means diameter of pore [32]. The rate of active release can also be controlled through diffusion or any other triggers such as pH, temperature, pressure or moisture [33].
Release triggers
The release trigger is defined as a mechanism, which can be used to release or accelerate active agents from Micro sponge Delivery System (MDS) [34].
Micro sponges can be designed to release a given amount of active ingredients over time in response to one or more external triggers [35].
i. Pressure triggered systems
Micro sponge system releases the entrapped material pressure/rubbing applied can release active ingredient from micro sponges onto skin. The amount released depends upon different characteristics of the sponge [36]. By varying the type of material and different process variables, the micro sponge best suited for a given application may be optimized. When compared with mineral oil containing micro capsules, mineral oil containing micro sponge showed much more softening effect. The emollient property duration was also much more for the micro sponge systems [37].
ii. Temperature changed triggered systems
Some entrapped active ingredients can be too viscous at room temperature to flow spontaneously from micro sponges onto the skin. Increased in skin temperature can result in an increased flow rate and hence release [38]. So, it is possible to modulate the release of substances from the micro sponge by modulation of temperature. For example, viscous sunscreens were found to show a higher release from micro sponges when exposed to higher temperatures; thus, a sunscreen would be released from a micro sponge only upon exposure to the heat from the sun [39].
iii. pH triggered systems
The pH-responsive micro sponges involve the coating of Conventional Micro sponge delivery systems with the enteric-coating type of material, which imparts pH responsiveness to this delivery system. Triggering the pH-based discharge of the active can be achieved by adapting the coating on the micro sponge [40].
iv. Solubility triggered systems
Micro sponges loaded with water-soluble ingredients like antiperspirants and antiseptics will release the ingredient in the presence of water [41]. Presence of an aqueous medium such as perspiration can trigger the release rate of active ingredients. Thus, release may be achieved based on the ability of the external medium to dissolve the active, the concentration gradient or the ability to swell the micro spore network [42].
Preparation of micro sponge
Preparation of Micro sponge is a complex process and involves plethora amount of time and money, the major step in preparation of micro sponge is Drug-Loading. In the micro sponges the drug loading can be done in two ways with one-stage or two-stage process; for loading on the basis of physical-chemical properties of the drug. If the drug is usually an inactive non polar material, then it makes the porous structure fashionable, which is called Porogen. Porogen drug, which neither interrupts polymerization nor activates it and stable for free radicals is entrapped with one-step process [43].
Ideal property of material to be incorporated in micro sponge
Material must be either fully miscible in monomer or capable of being made miscible by addition of small amount of co solvent. Material should be water immiscible or only slightly soluble. Material should be inert to monomers. Material should be stable in contact with polymerization catalyst and conditions of polymerization [44].
Method of preparation
Liquid – liquid suspension polymerization
Principle
The liquid-liquid suspension polymerization is method used for preparation of micro sponge. Polymerization done in the round bottom flask of polystyrene or Methyl methacrylate. In their preparation, Non-polar active constituents are dissolved with monomer in a suitable solvent instantly and then dispersed in aqueous phase this comprises of additives such as surfactant, suspended agents, etc. assistance in the formation of suspension. Once identified with the different droplets of the desired size after the suspension, polymerization is triggered by monomer stimulant or increased temperature or radiation [45].
Process
In this method two phases are mixed one phase contains active ingredient and monomer are mixed together to form a solution (which is non polar). This phase is suspended in second phase containing additives like surfactant or dispersing agents. Once the suspension is obtained with desired particle size polymerization is done by activating monomer either by increased temperature, irradiation or catalysis [46].
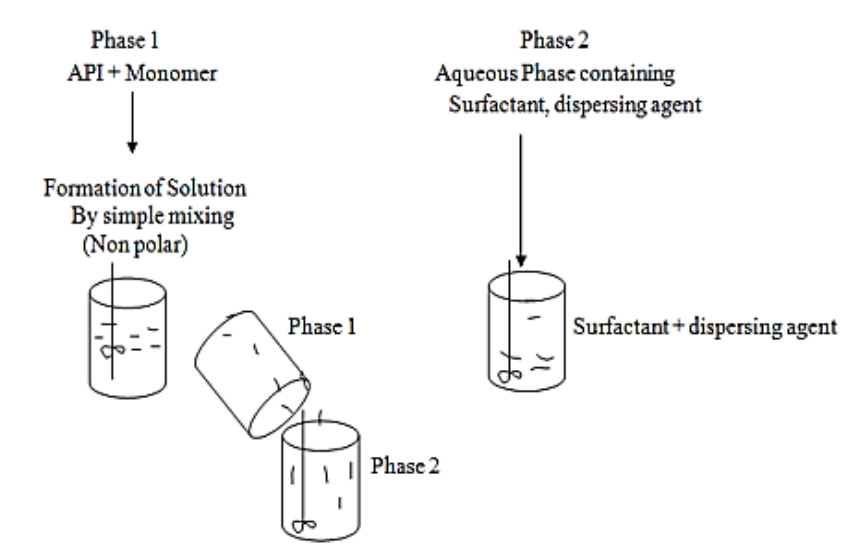
Fig. 5(a): Process in liquid-liquid suspension polymerization [46]
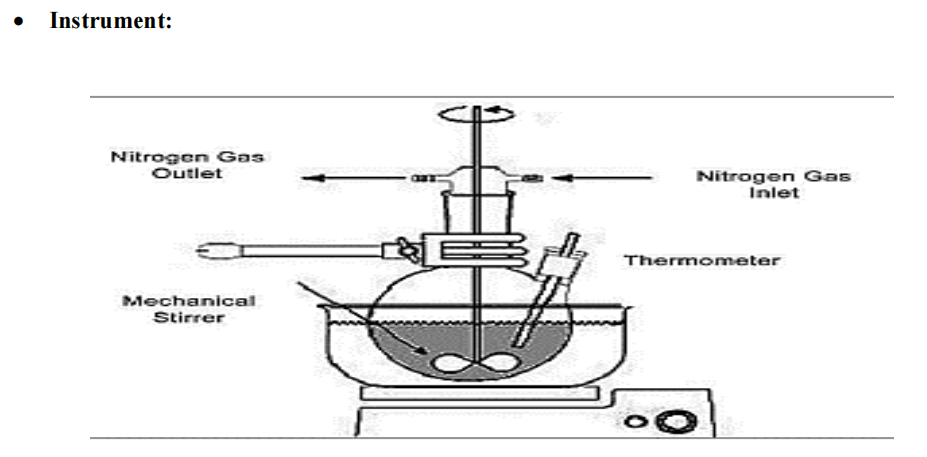
Fig. 5(b): Instrumentation of liquid-liquid suspension polymerization [45]
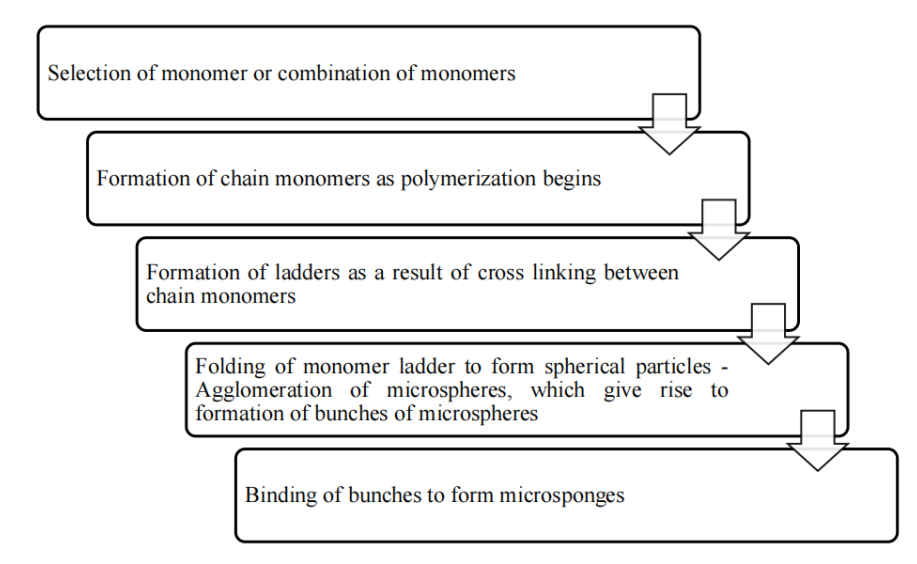
Fig. 6: The various steps involved are as follows [47]
Quasi – emulsion solvent diffusion
Principle
Quasi-emulsion solvent diffusion technique is most common method of preparation of microsponge. It is a two-step process, in which internal phase consisting of a suitable polymer is dissolved in solvents such as Dichloromethane, acetone or ethanol, in the presence of a plasticizer and a diffusible substance (Porogen). This internal phase is, then, dispersed into an external aqueous phase, comprising of polyvinyl alcohol, which acts as a stabilizer. After emulsification, the system is continuously stirred for a suitable time interval and maintained at a high temperature, if needed. Porogen diffuses into the external medium, resulting in a highly porous scaffold structure called ‘Micro sponge’. The final product is subsequently washed and dried in vacuum oven at 60 °C for 24 h [48].
Advantages over one step process
This process might prove a better option when the active molecule is sensitive to polymerization conditions. Further, the process has the advantage of avoiding solvent toxicity. Importantly, some factors such as drug solubility, nature of solvent, temperature and speed of emulsification, nature of polymer cross-linking, diffusivity of porogen, type and concentration of plasticizer also affect the formation of micro sponges. This process, besides being rapid, is simple and reproducible. Furthermore, this technique yields uniform microsponges with narrow size distribution [48].
Process
All micro sponges were prepared by a quasi-emulsion solvent diffusion method using an external phase of containing 200 ml distilled water and 40 mg polyvinyl alcohol (PVA) [47]. The internal phase consisted of ketoprofen, ethyl alcohol, polymer and triethyl citrate (TEC), which was added at an amount of 20% of the polymer in order to facilitate the plasticity. At first, the internal phase was prepared at 60 °C and added to the external phase at room temperature. After emulsification, the mixture was continuously stirred for 2 h. Then the mixture was filtered to separate the micro-sponges. The product was washed and dried by vacuum oven at 40 °C for 24 h [49].
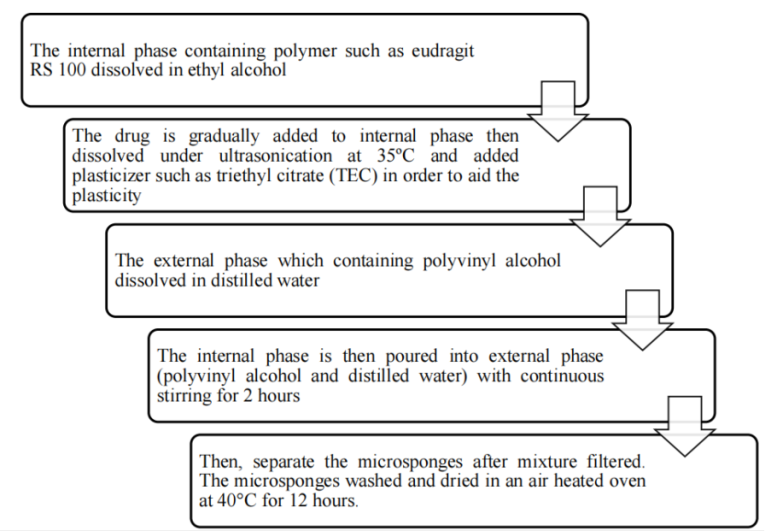
Fig. 7: The various steps involved are as follows [50]
Polymerization
Principle
Suspension polymerization method used for preparation of Porous Micro sponge in liquid-liquid systems. In their preparation, the monomers are first dissolved along with active ingredients in a suitable solvent solution of monomer and are then dispersed in the aqueous phase, which consist of additives (surfactant, suspending agents, etc. to aid in formation of suspension) [51]. The polymerization is then initiated by adding catalyst or by increasing temperature or irradiation. The various steps in the preparation of micro sponges are summarized as:-Selection of monomer or combination of monomers-Formation of chain monomers as polymerization begins-Formation of ladders as a result of cross linking between chain monomers-Folding of monomer ladder to form spherical particles-Agglomeration of micro spheres. The polymerization process leads to the formation of a reservoir type of system, which opens at the surface through pores. In some cases, an inert liquid immiscible with water but completely miscible with monomer is used during the polymerization to form the pore network. After the polymerization the liquid is removed leaving the porous micro spheres, i. e., microsponges. Impregnating them within preformed micro sponges then incorporates the functional substances. Sometimes solvent may be used for faster and efficient incorporation of the active substances. The micro sponges act as a topical carrier for variety of functional substances like, anti-acne, anti-inflammatory, antipruritics, anti-fungal, rubefacients, etc. [52]
Emulsion solvent diffusion method
In this method 2 phases are used in different proportion of organic and aqueous (ethyl cellulose and polyvinyl alcohol). The dispersed phase having ethyl cellulose and drug get dissolved in Dichloromethane (20 ml) and a definite amount of polyvinyl alcohol added to 150 ml of aqueous continuous phase. Then, the mixture is stirred properly at 1000 rpm for 2hr. The required micro sponges were collected by the process of filtration and kept for drying in oven at 40 °C for 24h. Micro sponges which are dried were stored in desiccators and ensure the removal of residual solvents is done [53].
Microsponge prepared from hyper – cross linked β-cyclodextrins
Prepared from β-Cyclodextrin act as micro porous materials performed their work as carriers for drug delivery. Due to these 3-D networks are formed which may be a roughly spherical structure about the size of a protein having channels and pores in the internal part. Reacting cyclodextrin with a cross linker such as diisocyanates, diaryl carbonates, carbonyl diimidazoles etc. [54]. Sponge size is controlled according to porosity, surface charge density for the attachment to different molecules. Micro sponges are synthesized in neutral or acidic form. They consist of solid particles and converted in crystalline form. Capacity of micro sponges to encapsulate drug having different structures and solubility. They are used to increased aqueous solubility of poorly-water soluble drugs [55].
Table 2: Factors affecting physical properties of microsponge
| Process variables | |
|
Production yield increases due to increase in mean size. Production yield decreases due to turbulence created within the external phase and reduction in mean size [56]. |
|
Production yield is directly responsible for quality and quantity of micro sponge produced [57]. |
|
Drug release can be affected by diffusion. It occurs due to the partition coefficient of the ingredient between the Micro sponge polymer and the outside medium [58]. |
|
Rubbing action on skin accelerates the release of the entrapped ingredient. Thus, facilitate the ingredients to pass via pores, channels or membranes. This action results in renewed product efficacy [59] |
|
Some API are sensitive to change in temperature, can be too viscous at room temperature to flow spontaneously from the Micro sponge onto the skin. When warmed by the skin temperature, the sun or other heat source, their viscosity may decrease, resulting in an increased flow rate [60] |
|
Particle size plays an important role in drug release from microsponge. Particles larger than 30μm can impart gritty feeling and hence particles of sizes between 10 and 25μm are preferred to use in final topical formulation. [61]. |
|
Pore or Channel size and structure affect the movement of active ingredients from microsponges into the vehicle in which the material is dispersed. Pore volume and diameter plays an important role in controlling the intensity and duration of effectiveness of the active ingredient [62] |
|
Micro sponges with varying Resiliency (viscoelastic) properties can be used to produced Bead lets that is softer or firmer according to the needs of the final formulation [63]. The degree of cross-linking affects the drug release from the prepared micro sponges, where increased cross-linking tends to decrease the release rate. Hence, viscosity measurements should be done so that the viscoelastic properties of micro sponges can be modified and adjusted to obtain the desired release properties [64] |
| Formulation variables | |
|
Particle size of Micro sponge were directly proportional to the apparent viscosity of the dispersed phase. Larger the difference between apparent viscosity of dispersed and continuous phase larger the mean particle size. When the dispersed phase with higher viscosity is poured into the continuous phase with lower viscosity, the globules of the formed emulsion can hardly be divided into smaller particles and bigger droplets are formed resulting in an increase in mean particle size [65]. Good Micro sponges can be produced only when 3 to 6 ml of internal phase is used A decrease in volume of external phase (water) results in decrease in production yield, mean particle size and drug content [66]. |
|
The polymer composition can affect partition coefficient of the entrapped drug between the vehicle and the micro sponge system which has a direct influence on the release rate of entrapped drug. It can be studied by plotting cumulative % drug release against time. The choice of monomer is dictated both by the vehicle into which it will be dispersed and characteristics of active ingredient to be entrapped. Polymers with varying degrees of hydrophobic or lipophilic or electrical charges may be prepared to give flexibility in the release of active ingredients. A variety of probable monomer combinations will be screened for their appropriateness with drugs by studying their drug release profile [67]. |
|
Production yield increases due to decrease in mean particle size. On the other hand, production yield decreases due to increase in mean particle size as well as non-ionic nature of emulsifier [64]. |
|
Increase in drug: polymer ratio leads decreased drug release. Due to the fact that the polymer amount available for each micro sponge for drug encapsulation is higher, thus, leading to more pronounced polymer matrix wall thickness. It results in extended diffusion path, and ultimately, to lesser drug release [65]. |
|
Enhance the entrapment efficiency. Also, pore inducers are hydrophilic in nature so it increases drug release. |
|
When the amount of Dichloromethane is increased from 5 to 15 ml the production yield and drug content of Micro sponges were found to be decreased which is due to the lower concentration of the drug in the higher volume of internal phase (i. e., dichloromethane) [64]. Dichloromethane concentration was also found to have a positive impact on the drug release. This is attributed to the fact that with increase in Dichloromethane concentration, more porous and spongy micro structures are obtained. Higher amount of Dichloromethane also results in the precipitation of the drug at the periphery of the micro sponge, leading to extended drug release [65]. |
|
The aqueous solubility should be optimum otherwise, the continuous phase will deplete the micro sponge during formulation, and polymer design and payload of micro sponges for the action must be optimized for required release after a given time period the solubility of actives in the vehicle is must be limited [66]. Otherwise, the vehicle will deplete the active ingredient before the application. To prevent cosmetic problems; not more than 10 to 12% w/w micro sponges must be incorporated into the vehicle [69]. By taking into account the solubility of the entrapped ingredient, the Micro sponges can be programmed to respond to water, perspiration or other solvents. An antiperspirant or antiseptic can thus be made to release its active material from a dry Micro sponges in the presence of water [66] |
Evaluation parameters
Particle size distribution
The particle size distribution is evaluated with the help of optical or electron microscope [56]. The size of the particles affects the consistency of the formulation and its stability. Particles larger than 30 μm can impart grittiness and hence particles of sizes between 10 and 25 μm are preferred in topical preparations [70].
Particle size was determined using optical microscopy at 10x and 40x. The microsponges were placed on glass slide and observed under optical microsponge. The size of 50-100 micro sponges was measured using optical microscope [71].
Morphology and surface topography of micro sponges
The occurrence of pores is an essential feature of micro sponges, its internal and external morphology, and surface topography can be obtained by using scanning electron microscopy and transmission electron microscopy [72].
Scanning electron microscopy (SEM) is an electron optical imaging technique that provides photographic images and elemental information about prepared micro sponges [73]. SEM is useful for characterizing the morphology and size of microscopic specimens with particle size as low as nano-metre to decametre, the sample is placed in an evacuated chamber and scanned by an electron beam in a controlled pattern [74]. When the electron beam interact with the specimen produces a variety of physical phenomena that when detected are used to form images and provide elemental information about the specimens. Micro sponge were fixed on aluminium studs and coated with gold using a sputter coater SC 502, under vacuum [0.1 mm Hg]. The micro sponge were then analyzed by scanning electron microscopy (SEM) [70, 72].
Characterization of pore structure
Pore volume and pore diameter are climacteric in controlling the intensity as well as duration of effectiveness of the active ingredient. Pore diameter can also affect the passage of active ingredients from micro sponges into the vehicle in which the material is dispersed. The effect of pore diameter as well as volume with rate of drug release from micro sponges can be studied by mercury intrusion Porosity. Porosity parameters of micro sponges such as intrusion– extrusion isotherms, total pore surface area, pore size distribution, average pore diameters, shape and morphology of the pores, bulk and apparent density can also be determined by using mercury intrusion porosity [75]. In this test, a sample of micro sponges is placed in a vacuum chamber and submerged under a mercury pool, in a volume-calibrated cell. When the pressure is gradually increased in the cell, mercury is forced into pores of micro sponges. This leads to reduction in the apparent volume of mercury within the calibrated cell [76]. Elevated intrusion volumes can be plotted against pore diameters that represented pore size distributions.
• The pore diameter of micro sponges can be calculated by using Wash burn equation
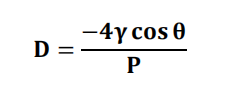
Where,
D = pore diameter (m)
Surface tension of mercury = 485 dyne cm−1
The contact angle = 130°
P = Pressure (psia).
![]()
• Pore morphology can be characterized from the intrusion–extrusion profiles of mercury in the Micro sponges.
Powder X-ray diffraction (PXRD)
Powder X-ray diffraction is a valuable analysis method to study physio-chemical features of crafted micro sponges. XRD pattern of the micro sponge can be determined as a function of scattered angles due to dispersion of atoms in their lattice planes [77]. Powder X-ray diffraction has been used for assessing the changes in crystalline form of drug and chemical interaction between the ingredients in micro sponges [78]. PXRD analysis also gives information about thermal stability of drug in micro sponge formulation. X-ray powder diffraction of micro sponge were analyzed by Philips PW 1729 x-ray refractometer. Samples were irradiated with monochromatic Cu Kα –radiations (1.542 Å) and analyzed between 2-60° (2θ). The voltage and current used were 30kV and 30 mÅ respectively. The range was 5 x 103 cycles/s and the chart speed were kept at 100 mm/2θ [79].
Production/Percentage yield
The production yield of the micro sponges can be obtained by calculating accurately the initial weight of the raw materials and the last weight of the micro sponge.
The dried micro sponges of each batch are weight separately and percentage yield is calculated by using following equation: [74]
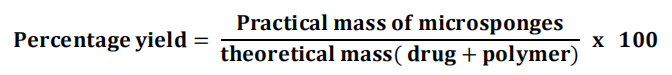
Entrapment efficiency
Entrapment efficiency is the content of core material effectively entrapped in a formulation. Entrapment efficiency is influenced by several parameters: drug polymer ratio and amount of pore inducers [74]. Increase in the drug-polymer ratio may initiate reduction in diffusion rate of drug solution from concentrated polymeric solutions into the external phase [79]. Thus, more time for droplet formation, resulting in enhancement of micro sponge yield and entrapment efficiency. This maybe stemmed from the fact that the amount of drug per unit of polymer is more [72]. It can be measured by an indirect method in which micro sponge suspension can be centrifuged at 2000 rpm for 10 min. The supernatant obtained can be suitably diluted with suitable solvent and the amount of free drug present in supernatant can be quantified using UV– Visible spectroscopy or HPLC. The drug entrapment efficiency can be calculated using the following formula [74].
![]()
Total drug content
• The total drug content (TDC) calculated as given below:
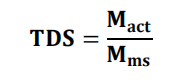
Where, Mact = actual amount of drug in weighed quantity of micro sponges.
Mms = the weighed quantity of micro sponges [66].
In vitro drug release
In vitro release studies have been carried out using dissolution apparatus USP XXIII equipped with a modified basket consisted of 5µm stainless steel mesh. Dissolution rates were measured at 37 °C under 150 rpm rotor speed [74]. The dissolution medium is selected while considering solubility of active ingredients to ensure sink conditions. Sample aliquot were withdrawn from the dissolution medium and analyzed by suitable analytical method (UV spectra-photometer) at regular intervals of time [78].
Kinetics of drug release
The results of in vitro release profile obtained for all the formulations were plotted in kinetic models as follows, a) cumulative of drug released versus time (zero order kinetic models). b) Log cumulative percent drug remaining to be absorbed versus time (1st order model) c) Cumulative amount of drug release versus square root of time (Higuchi model equation) d) Log cumulative drug release versus log time (Korsmeyer – Pep pas model) [74, 77].
Determination of true density
True density can be measured by an ultra-pycnometer using helium gas, and calculated as a mean of repeated determinations [74, 75].
Compatibility studies
The drug-excipients compatibility studies are carried out in order to ensure that there is no inadvertent reaction between the two when formulated into a dosage form [68]. These studies are commonly carried out by recording the DSC of drug and excipients individually and also together and checking for any addition or deletion of any peaks or troughs. Compatibility of drug with reaction adjuncts can also be studied by thin layer chromatography (TLC) and FTIR [80].
Effect of polymerization on crystallinity of the drug can be studied by powder X-ray diffraction (XRD) and Differential scanning colorimetry (DSC). For DSC, approximately 5 mg sample can be weighed accurately into aluminum pans, then sealed and can be run at a heating rate of 15 °C/min over a temperature range 25–430 °C in atmosphere of nitrogen [72, 80].
Stability studies
Micro sponges remain stable in the range of physiological pH and due to their property of thermostability, they can withstand temperatures up to 130 °C. As their average pore size is 0.25 μm, micro sponge formulations are self-sterilizing, limiting bacterial penetration. Although, microbial entry to the bulk is prevented, they can grow on the surface of micro sponges [44]. The stability studies of micro sponges were carried out in accelerated conditions as per ICH guidelines. The micro sponge formulations were kept at 40 °C±2 °C and 75%±5% RH for 3 mo. After 3 mo, micro sponges were analyzed for physical appearance, in vitro drug release and FTIR spectroscopy [81].
Safety considerations
The preparation methods usually use organic solvents as porogens, which pose an environmental hazard, as some may be highly inflammable, posing a safety hazard. In some cases, the traces of residual monomers have been observed, which may be toxic and hazardous to health. Skin irritation studies in rabbits the scores for erythema totaled for intact and abraded skin for all rabbits at 24 and 72 h. The primary irritation index was calculated based on the sum of the scored reactions divided by 24 (two scoring intervals multiplied by two test parameters multiplied by six rabbits) [81]. Safety studies of micro sponges can be confirmed by Allergenicity in guinea pigs, eye irritation studies in rabbits, Mutagenic in bacteria, Oral toxicity studies in rats and skin irritation studies in rabbits [82].
The true worth of a delivery system is judged on the basis of its ability to deliver effective concentration of the active agent without compromising on the safety aspects. In other words, the drug should be released from the delivery system in such a manner that it does not induce any irritation, cyto-toxicity, geno-toxicity or immunogenicity [72]. Cytotoxicity evaluation of the drug loaded microporous carriers constitutes an important aspect of formulation development. The cytotoxic effects of both, the active agent as well as the ingredients comprising the delivery system, should be taken into account [76].
Applications
Micro sponge delivery systems are used to enhance the safety, effectiveness and aesthetic quality of topical prescription, over-the-counter and personal care products. It is used mostly for topical and recently for oral administration [82]. Several patents have reported that it can be used as excipients due to its high loading capacity and sustained release ability. It offers the formulator a range of alternatives to develop drug and cosmetic products. Micro sponges are designed to deliver a pharmaceutical active ingredient efficiently at the minimum dose and also to enhance stability, reduce side effects and modify drug release. Over-the-counter products that incorporate micro sponge drug delivery system include numerous moisturizers, specialized rejuvenate products, and sunscreens.
Micro sponge drug delivery systems offer entrapment of ingredients and is believed to contribute towards reduced side effects, improved stability, reduces systemic exposure and minimize local cutaneous reactions, increased elegance, and enhanced formulation flexibility. One of the most important features of micro sponge is their ability to absorb skin secretions, i. e., oil and sweat [72]. Due to their highly absorbent nature, many micro sponge loaded deodorants, antiperspirants and sunscreens are commercially available. Further, micro sponge drug delivery systems can be used for skin targeting, avoiding excessive absorption of drug into the percutaneous blood circulation. This feature may prove a boon in skin disorders, like skin cancer, wounds, acne, Alopecia, sunburn, hyperhidrosis and wrinkles [74].
Microsponge for topical delivery
The Micro sponge systems are based on microscopic, polymer-based micro spheres that can bind, suspend or entrap a wide variety of substances and then be incorporated into a formulated product, such as a gel, cream, liquid or powder [8]. Like a true sponge, each micro sphere consists of a myriad of interconnecting voids within a non-collapsible structure that can accept a wide variety of substances. The outer surface is typically porous, allowing the controlled flow of substances into and out of the sphere. Several primary characteristics, or parameters, of the micro sponge system can be defined during the production phase to obtain spheres that are tailored to specific product applications and vehicle compatibility [8]. Benzyl peroxide is commonly used in topical formulations for the treatment of acne and athlete’s foot. Skin irritation is a common side effect, and it has been shown that controlled release of Benzyl peroxide from a delivery system to the skin could reduce the side effect while reducing percutaneous absorption [82].
Table 2: Some examples of topical products [83]
| Product | Advantages | Examples | Reference |
| Sunscreens | Long lasting product efficacy, with improved protection against sunburns and sun related injuries even at elevated concentration and with reduced irritation and sensitization. | Hydroquinone, Retinol | [83] |
| Anti-acne | Maintained efficacy with decreased skin irritation and sensitization. | Benzoyl peroxide | [84] |
| Anti-inflammatory | Long lasting activity with reduction of skin allergic response and dermatitis. | Diclofenac sodium, hydrocortisone | [85] |
| Anti-dandruff | Reduced unpleasant odour with lowered irritation with extended safety and efficacy | zinc pyrithione, selenium sulfide | [86] |
| Anti-pruritic | Extended and improved activity | Benzoyl peroxide | [87] |
| Rubefacients | Prolonged activity with reduced irritation greasiness and odor. | Salicylates, capsaicin | [88] |
| Skin depigmentation | Improved stabilization against oxidation with improved efficacy and aesthetic appeal. | hydroquinone | [89] |
Microsponge for oral delivery
A micro sponge system offers the potential to hold active ingredients in a protected environment and provide controlled delivery of oral medication to the lower gastrointestinal (GI) tract, where it will be released upon exposure to specific enzymes in the colon. This approach if successful should open up entirely new opportunities for micro sponge. In oral applications, the micro sponge system has been shown to increase the rate of solubility of poorly water-soluble drugs by entrapping such drugs in the micro sponge system's pores. Because these pores are very small, the drug is in effect reduced to microscopic particles with resultant increase in surface area and thus greatly increases the rate of solubility. An added benefit is that the time it requires for micro sponge system to traverse the small and large intestine is significantly increased thus maximizing the amount of drug that is absorbed Ketoprofen was used as a model drug for systemic drug delivery of micro sponges in the study. Ketoprofen micro sponges were prepared by quasi-emulsion solvent diffusion method with Eudragit RS 100 and afterwards tablets of micro sponges were prepared by direct compression method. Different pressure values were applied to the tablet powder mass in order to determine the optimum pressure value for compression of the tablets. Result indicated that compression was much improved over the physical mixture of the drug and polymer; due to the plastic deformation of sponge-like structure, micro sponges produce mechanically strong tablets [90].
Applications of micro sponge in bone and tissue engineering
Micro sponge drug delivery systems are being explored for bone and tissue engineering applications due to their ability to deliver drugs or growth factors efficiently and promote bone regeneration and tissue repair [91].
Table 3: List of marketed products using micro sponge drug delivery system
| S. No. | Product name | Uses | References |
| 1 | Retin-A-Micro | 0.1% and 0.04% Tretinoin entrapped in Micro sponge for topical treatment of acne vulgaris. This formulation uses patented Methyl methacrylate/glycol dimethacrylate cross-polymer porous micro spheres (Micro sponge System) to enable inclusion of the active ingredient, tretinoin, in an aqueous gel. | [92] |
| 2 | Carac Cream, 0.5% | Carac cream contains 0.5% fluorouracil, with 0.35% being incorporated into a patented porous micro sphere (Micro sponge) composed of methyl methacrylate/glycol dimethacrylate cross-polymer and di-methicone. Carac cream is a once-a-day topical prescription product for the treatment of actinic or solar keratitis. | [93] |
| 3 | Line Eliminator Dual Retinol Facial Treatment | Lightweight cream with a retinol in Micro sponge delivers both immediate and time released wrinkle fighting action. | [94] |
| 4 | Retinol cream | The retinol molecule is kept in the micro sponge system to protect the potency of the vitamin A by reducing the possibility of irritation | [95] |
| 5 | Retinol 15 Night cream | A nighttime treatment cream with Micro sponge technology using a stabilized formula of pure (visible diminish of fine lines and wrinkles. | [96] |
| 6 | EpiQuin Micro | The Micro sponge system entrapping hydro-quinone and retinol release drug into the skin gradually throughout the day (minimize skin irritation) | [97] |
| 7 | Sports-cream RS and XS | Topical analgesic-anti-inflammatory and counter-irritant actives in a Micro sponge Delivery System for the management of musculoskeletal conditions | [98] |
CONCLUSION
The micro sponge delivery system is a unique technology. It is widely applied in the pharmaceutical and cosmetic industries due to its ability to deliver drugs in a controlled and efficient manner. It enable the controlled release of active ingredients, leading to a reduction in side effects and improved therapeutic efficacy. When it is applied in the form of micro sphere it can improve formulation stability. Studies suggest that micro sponge systems are generally non-toxic, non-allergenic, and non-mutagenic. Moreover, it can be applied in bone and tissue engineering. Thus, it can be exploited to deliver drug to treat various diseases in future.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
Satyajit Sahoo was involved in drafting and writing related manuscript. Tejas Patel and Sapna Desai contributed to literature survey of the topic. Sohan Patel has undergone the review and validated. All authors read and approved the final manuscript.
CONFLICT OF INTERESTS
The authors declare no conflict of interest.
REFERENCES
Park H, Otte A, Park K. Evolution of drug delivery systems: from 1950 to 2020 and beyond. J Control Release. 2022 Feb;342:53-65. doi: 10.1016/j.jconrel.2021.12.030, PMID 34971694.
Ezike TC, Okpala US, Onoja UL, Nwike CP, Ezeako EC, Okpara OJ. Advances in drug delivery systems challenges and future directions. Heliyon. 2023 Jun 24;9(6):e17488. doi: 10.1016/j.heliyon.2023.e17488, PMID 37416680.
Alshammari ND, Elkanayati R, Vemula SK, Al Shawakri E, Uttreja P, Almutairi M. Advancements in colon targeted drug delivery: a comprehensive review on recent techniques with emphasis on hot melt extrusion and 3D printing technologies. AAPS PharmSciTech. 2024;25(7):236. doi: 10.1208/s12249-024-02965-w, PMID 39379609.
Adepu S, Ramakrishna S. Controlled drug delivery systems: current status and future directions. Molecules. 2021;26(19):5905. doi: 10.3390/molecules26195905, PMID 34641447.
Eugster R, Luciani P. Liposomes: bridging the gap from lab to pharmaceuticals. Curr Opin Colloid Interface Sci. 2025 Feb;75:101875. doi: 10.1016/j.cocis.2024.101875.
Saxena S, Nacht S. Delivery system handbook for personal care and cosmetic products: technology applications and formulations. New York: William Andrew Publishing; 2005. p. 333-51.
Kaity S, Maiti S, Ghosh AK, Pal D, Ghosh A, Banerjee S. Microsponges: a novel strategy for drug delivery system. J Adv Pharm Technol Res. 2010 Jul;1(3):283-90. doi: 10.4103/0110-5558.72416, PMID 22247859.
Srinatha N, Battu S, Vishwanath BA. Microsponges: a promising frontier for prolonged release current perspectives and patents. Beni Suef Univ J Basic Appl Sci. 2024;13(1):60. doi: 10.1186/s43088-024-00519-4.
Won R. Method for delivering an active ingredient by controlled time release utilizing a novel delivery vehicle which can be prepared by a process utilizing the active ingredient as a porogen; 1987.
Naga Jyothi K, Dinesh Kumar P, Arshad P, Karthik M, Panneerselvam T. Micro sponges: a promising novel drug delivery system. J Drug Deliv Ther. 2019 Oct;9(5):188-94. doi: 10.22270/jddt.v9i5-s.3649.
Biswas R, Saha S, Chatterjee C, Ghosh S. Micro sponges: a promising approach for drug delivery. Int J Pharm Sci Res. 2022;13(1):42-9. doi: 10.13040/IJPSR.0975-8232.13(1).42-49.
Kaity S, Maiti S, Ghosh AK, Pal D, Ghosh A, Banerjee S. Microsponges: a novel strategy for drug delivery system. J Adv Pharm Technol Res. 2010;1(3):283-90. doi: 10.4103/0110-5558.72416, PMID 22247859.
Ingale DJ, Aloorkar NH, KulkarnI AS, Patil RA. Microsponges as innovative drug delivery systems. PCI Approved IJPSN. 2012;5(1):1597-606. doi: 10.37285/ijpsn.2012.5.1.2.
Bhimavarapu R, Devi RR, Nissankararao S, Devarapalli C, Paparaju S. Micro sponges as a novel imperative for drug delivery system. Res J Pharm Technol. 2013;6(8):842-8.
Saraf A, Dasani A, Pathan HK. Micro sponge drug delivery system as an innovation in cosmetic world: a review. Asian J Pharm Educ Res. 2012;1(2):67-87. doi: 10.22270/jddt.v11i1.4492.
Choudhary A, Akhtar MS. Microsponge drug delivery system: emerging technique in novel drug delivery system and recent advances. Res J Pharm Technol. 2022;15(10):4835. doi: 10.52711/0974-360X.2022.00812.
Vishwakarma P, Choudhary R. Micro sponges: a novel strategy to control the delivery rate of active agents with reduced skin irritation. J Drug Deliv Ther. 2019;16(6):247. doi: 10.22270/jddt.v9i6-s.3757.
Shaw A, Gaur N, Mishra S. Micro sponges a novel approach for drug targeting. Int J Pharm Res. 2020;12:SP1.327. doi: 10.31838/ijpr/2020.
Kawashima Y, Niwa T, Takeuchi H, Hino T, Ito Y. Control of prolonged drug release and compression properties of ibuprofen microspheres with acrylic polymer eudragit RS by changing their intraparticle porosity. Chem Pharm Bull (Tokyo). 1992 Jan 25;40(1):196-201. doi: 10.1248/cpb.40.196, PMID 1576674.
Singh S, Gupta MK, Devi A. Development and characterization of micro sponge gel containing piperine for antibacterial. J Popul Ther Clin Pharmacol. 2024;31(6):1864-80. doi: 10.53555/jptcp.v31i6.6774.
Mahant S, Kumar S, Nanda S, Rao R. Microsponges for dermatological applications: perspectives and challenges. Asian J Pharm Sci. 2020;15(3):273-91. doi: 10.1016/j.ajps.2019.05.004, PMID 32636947.
Neta A, Bajaj A, Madan M. Development of micro sponges for topical delivery of mupirocin. AAPS PharmSciTech. 2009;10(2):123-8.
V, Rani R, Singh AP, Singh AP. Micro sponge as novel drug delivery system. International Journal of Pharmaceutics and Drug Analysis. 2024 Jun;12(2):14-8. doi: 10.47957/ijpda.v12i2.584.
Patel S, Shah C, Upadhyay U. A review on micro sponges drug deliv syst. International Journal of Pharmaceutical Research and Applications 2024;9(2):2244-51. doi: 10.35629/4494-090222442251.
Kaity S, Maiti S, Ghosh AK, Pal D, Ghosh A, Banerjee S. Microsponges: a novel strategy for drug delivery system. J Adv Pharm Technol Res. 2010 Jul;1(3):283-90. doi: 10.4103/0110-5558.72416, PMID 22247859.
Sawant SS, Patil SS, Kandle HS, Kengar MD, Vambhurkar GB, Bhutkar MA. Development and characterization of lornoxicam loaded microsponge gel for rheumatoid arthritis. Asian J Pharm Technol. 2019;9(3):173-8. doi: 10.5958/2231-5713.2019.00029.1.
Valluru R, Ravi G, Bose SP, Damineni S. Micro sponges a comprehensive review: success and challenges. Indo Am J Pharm Res. 2019;9(7):3056-67. doi: 10.5281/zenodo.3356512.
Pawan AS, Prashant PB. A new era in topical formulations micro sponge drug delivery system. Int J Pharm Sci Res. 2016 Jul 1;7(7):2756. doi: 10.13040/IJPSR.0975-8232.7(7).2756-61.
Ingale DJ, Aloorkar NH, KulkarnI AS, Patil RA. Microsponges as innovative drug delivery systems. IJPSN. 2012;5(1):1597-606. doi: 10.37285/ijpsn.2012.5.1.2.
Tiwari A, Mishra MK, Shukla A, Yadav SK. Micro sponge: an augmented drug delivery system. Am J Pharm Tech Res. 2016;6(6):79-95.
Shrivastava S, Kumar D, Dubey CK, Singh SP, Khinchi MP. A review: micro sponge an effective drug delivery system. Asian J Pharm Res Dev. 2017 Mar 1:1-8.
Yadav V, Jadhav P, Dombe S, Bodhe A, Salunkhe P. Formulation and evaluation of micro sponge gel for topical delivery of the antifungal drug. Int J Appl Pharm. 2017;9(4):30-7. doi: 10.22159/ijap.2017v9i4.17760.
Embil K, Nacht S. The microsponge delivery system (MDS): a topical delivery system with reduced irritancy incorporating multiple triggering mechanisms for the release of actives. J Microencapsul. 1996;13(5):575-88. doi: 10.3109/02652049609026042, PMID 8864994.
Pentewar R, Kazi S, Bharti R, Pulgamwar G. MDS technology: an approach for topical oral controlled and cosmetic formulations. Res J Pharm Biol Chem Sci. 2014;5(3):1170-90.
Rajeswari S, Swapna V. Micro sponges as a neoteric cornucopia for drug delivery systems. Int J Curr Pharm Res. 2019;11(3):4-12. doi: 10.22159/ijcpr.2019v11i3.34099.
Rakhimol K, Biju P, Sindhoor SM, Aggarwal NN, VS, Rai D. Development of aceclofenac loaded micro sponge gels: a statistical quality by design (qbd) approach towards optimization and evaluation. Int J App Pharm. 2023;15(6):178-87. doi: 10.22159/ijap.2023v15i6.49122.
Sah S, Kohri A, Patil S. A comprehensive review on microsponges drug delivery systems. J Res Appl Sci Biotechnol. 2024;3(3):59-66. doi: 10.55544/jrasb.3.3.11.
Amrutiya N, Bajaj A, Madan M. Development of microsponges for topical delivery of mupirocin. AAPS PharmSciTech. 2009;10(2):402-9. doi: 10.1208/s12249-009-9220-7, PMID 19381834.
Chadawar V, Shaji J. Microsponge delivery system. Curr Drug Deliv. 2007 Apr;4(2):123-9. doi: 10.2174/156720107780362320, PMID 17456031.
Gangadharappa HV, Gupta NV, Prasad M SC, Shivakumar HG. Current trends in microsponge drug delivery system. Curr Drug Deliv. 2013;10(4):453-65. doi: 10.2174/1567201811310040010, PMID 22974222.
Tiwari A, Tiwari V, Palaria B, Kumar M, Kaushik D. Microsponges: a breakthrough tool in pharmaceutical research. Futur J Pharm Sci. 2022;8(1):31. doi: 10.1186/s43094-022-00421-9.
Kapoor D, Vyas RB, Lad C, Patel M, Tyagi BL. A review on micro-sponge drug delivery system. J Drug Deliv Ther. 2014;4(5):29-35. doi: 10.22270/jddt.v4i5.978.
Kumar R, Ashawat A, Jindal S. Microsponge for skin treatment: an updated review. Res J Top Cosmet Sci. 2024;15(1):6-12. doi: 10.52711/2321-5844.2024.00002.
Jyothi KN, Kumar PD, Arshad P, Karthik M, Panneerselvam T. Micro sponges: a promising novel drug delivery system. J Drug Deliv Ther. 2019 Oct 15;9(5):188-94. doi: 10.22270/jddt.v9i5-s.3649.
Srinatha N, Battu S, Vishwanath BA. Microsponges: a promising frontier for prolonged release current perspectives and patents. Beni Suef Univ J Basic Appl Sci. 2024;13(1):60. doi: 10.1186/s43088-024-00519-4.
Tyagi G, Sharratt WN, Erikson S, Seddon D, Robles ES, Cabral JT. Solution structures of anionic amphoteric surfactant mixtures near the two-phase region at fixed pH. Langmuir. 2022;38(23):7198-207. doi: 10.1021/acs.langmuir.2c00527, PMID 35658451.
Pentewar RS, Kazi S, Bharti R. Pulgamwar G. MDS technology: an approach for topical oral controlled and cosmetic formulations. Res J Pharm Biol Chem Sci. 2014;5(3):1170-90.
Mahant S, Kumar S, Nanda S, Rao R. Microsponges for dermatological applications: perspectives and challenges. Asian J Pharm Sci. 2020 May 1;15(3):273-91. doi: 10.1016/j.ajps.2019.05.004, PMID 32636947.
Tomar M, Pahwa S. Micro sponge drug delivery system. IJPSR. 2021;12(9):4697-707. doi: 10.13040/IJPSR.0975-8232.12(9).4697-07.
Chen H, Xu H, Wang C, Kang H, Haynes CL, Mahanthappa MK. Novel quasi-emulsion solvent diffusion based spherical cocrystallization strategy for simultaneously improving the manufacturability and dissolution of indomethacin. Cryst Growth Des. 2020;20(10):6752-62. doi: 10.1021/acs.cgd.0c00876.
Valluru R. Micro sponges a comprehensive review: success and challenges. Indo Am J Pharm Res. 2019;9(7):3056-67. doi: 10.5281/zenodo.3356512.
Florence AT. Targeted and controlled drug delivery: novel carrier systems. Int J Pharm. 2003;267(1-2):157. doi: 10.1016/S0378-5173(03)00356-9.
Sharma R, Roderick B, Pathak K. Evaluation of kinetics and mechanism of drug release from econazole nitrate micro sponges loaded carbopol hydrogel. Indian J Pharm Educ Res. 2011;45(1):25-31.
Setijadi E, Tao L, Liu J, Jia Z, Boyer C, Davis TP. Biodegradable star polymers functionalized with β-cyclodextrin inclusion complexes. Biomacromolecules. 2009 Sep 14;10(9):2699-707. doi: 10.1021/bm900646g, PMID 19663421.
Nidhi K, Verma S, Kumar S. Microsponge: an advanced drug delivery system. Journal of Clinical and Scientific Research. 2021;10(2):108-11. doi: 10.4103/JCSR.JCSR_42_19.
Mandal S, Km Bhumika B, Kumar M, Hak J, Vishvakarma P, Sharma UK. A novel approach on micro sponges drug delivery system: method of preparations application and its future prospective. Ind J Pharm Edu Res. 2023;58(1):45-63. doi: 10.5530/ijper.58.1.5.
Zaki Rizkalla CM, latif Aziz R, Soliman II. In vitro and in vivo evaluation of hydroxyzine hydrochloride microsponges for topical delivery. AAPS PharmSciTech. 2011;12(3):989-1001. doi: 10.1208/s12249-011-9663-5, PMID 21800216.
Yadav V, Jadhav P, Dombe S, Bodhe A, Salunkhe P. Formulation and evaluation of micro sponge gel for topical delivery of the antifungal drug. Int J App Pharm. 2017;9(4):30-7. doi: 10.22159/ijap.2017v9i4.17760.
Shinkar DM, Bhamare BS, Saudagar RB. Microsponges. Asian J Res Pharm Sci. 2016;6(2):77-84. doi: 10.5958/2231-5659.2016.00011.4.
Lendave AS. Microsponge technology for innovative drug delivery system. IJSRST. 2020;7(5):63-75. doi: 10.32628/IJSRST207521.
Thomas TS, N, Pa D, Carla B. Micro sponge drug delivery system for topical delivery a review. IJPCBS. 2016;6(4):424-31.
Tomar M, Pahwa S. Micro sponges drug delivery system. Int J Pharm Sci Res. 2021;12(9);4697-707. doi: 10.13040/IJPSR.0975-8232.12(9).4697-07.
Jyoti J, Kumar S. Innovative and novel strategy: microsponges for topical drug delivery. J Drug Delivery Ther. 2018;8(5):28-34. doi: 10.22270/jddt.v8i5.1885.
Halder S, Behera US, Poddar S, Khanam J, Karmakar S. Preparation of microsponge drug delivery system (MSDDS) followed by a scale-up approach. AAPS PharmSciTech. 2024;25(6):162. doi: 10.1208/s12249-024-02874-y, PMID 38997615.
Sah S, Kohri A, Patil S. A comprehensive review on microsponges drug delivery systems. J Res Appl Sci Biotechnol. 2024;3(3):59-66. doi: 10.55544/jrasb.3.3.11.
Kumar L, Chadha M, Rana R, Kukreti G, Kaundal AK, Aggarwal V. Polymeric microsponges: an emerging prospect in topical delivery of therapeutic agents. International Journal of Polymeric Materials and Polymeric Biomaterials. 2024;73(11):1003-19. doi: 10.1080/00914037.2023.2235872.
Soni S, Sisodiya D, Bhargava T, Dashora K. A microsponge based drug delivery system for the treatment of fungal infection. Int J Pharm Sci. 2024;2(4):664-79. doi: 10.5281/zenodo.10958379.
Jilta RK, Prashar D, Gupta A, Thakur P. Pharmaceutical and economical prospective of microsponges drug delivery system. Asian J Pharm Res Dev. 2025;13(1):81-3. doi: 10.22270/ajprd.v13i1.1507.
Adheena Jeby AJ, Ganesh NS GN, Adlin Jino Nesalin AJ, Chandy V. Unlocking the potential of microsponges: a versatile andfuturistic approach to oral drug delivery. Int J Pharm Res Appl. 2025;10(1):1059-68. doi: 10.35629/4494-100110591068.
Borawake PD, Kauslya A, Shinde JV, Chavan RS. Microsponge as an emerging technique in novel drug delivery system. J Drug Deliv Ther. 2021;11(1):171-82. doi: 10.22270/jddt.v11i1.4492.
Jyoti J, Kumar S. Innovative and novel strategy: micro sponges for topical drug delivery. J Drug Deliv Ther. 2018 Sep 6;8(5):28-34. doi: 10.22270/jddt.v8i5.1885.
Aldawsari H. Microsponges as promising vehicle for drug delivery and targeting: preparation characterization and applications. Afr J Pharm Pharmacol. 2013 May 8;7(17):873-81. doi: 10.5897/AJPP12.1329.
Oberemok SS, Shalita AR. Acne vulgaris I: pathogenesis and diagnosis. Pharmaceutics, New York; 2002 Aug 1;70(2):101-5. PMID 12234155.
Orlu M, Cevher E, Araman A. Design and evaluation of colon specific drug delivery system containing flurbiprofen microsponges. Int J Pharm. 2006 Aug 2;318(1-2):103-17. doi: 10.1016/j.ijpharm.2006.03.025, PMID 16687222.
Vinay J, Akanksha T. Carbohydrate polymers: applications and recent advances in delivering drugs to the colon. Carbohydr Polym. 2011 Jan;88(2):399-416. doi: 10.1016/j.carbpol.2011.12.021.
Moin A, Deb TK, Osmani RA, Bhosale RR, Hani U. Fabrication characterization and evaluation of microsponge delivery system for facilitated fungal therapy. J Basic Clin Pharm. 2016 Mar;7(2):39-48. doi: 10.4103/0976-0105.177705, PMID 27057125.
Osmani RA, Aloorkar NH, Ingale DJ, Kulkarni PK, Hani U, Bhosale RR. Microsponges based novel drug delivery system for augmented arthritis therapy. Saudi Pharm J. 2015;23(5):562-72. doi: 10.1016/j.jsps.2015.02.020, PMID 26594124.
Amrutiya N, Bajaj A, Madan M. Development of microsponges for topical delivery of mupirocin. AAPS PharmSciTech. 2009;10(2):402-9. doi: 10.1208/s12249-009-9220-7, PMID 19381834.
Kuppala RR, Prakash PR, Devanna N. Advancing topical posaconazole delivery: box behnken design micro sponge hydrogel optimization and extensive in vivo investigation. Int J Appl Pharm. 2024;16(6):238-43. doi: 10.22159/ijap.2024v16i6.51167.
Mahant S, Kumar S, Nanda S, Rao R. Microsponges for dermatological applications: perspectives and challenges. Asian J Pharm Sci. 2020 May 1;15(3):273-91. doi: 10.1016/j.ajps.2019.05.004, PMID 32636947.
Patel A, Upadhyay P, Trivedi J, Shah S, Patel J. Micro sponges as the versatile tool for topical route: a review. Int J Pharm Sci Res. 2012 Sep 1;3(9):2926. doi: 10.13040/IJPSR.0975-8232.3(9).2926-37.
Badhe KP, Saudagar RB. A review on microsponge a novel drug delivery system. Asian Journal Pharmaceutical Research. 2016;6(1):51-7. doi: 10.5958/2231-5713.2016.00008.8.
Jyothi KN, Kumar PD, Arshad P, Karthik M, Panneerselvam T. Micro sponges: a promising novel drug delivery system. J Drug Deliv Ther. 2019;9(5):188-94. doi: 10.22270/jddt.v9i5-s.3649.
Aldawsari H. Microsponges as promising vehicle for drug delivery and targeting: preparation characterization and applications. Afr J Pharm Pharmacol. 2013;7(17):873-81. doi: 10.5897/AJPP12.1329.
Rahman M, Almalki WH, Panda SK, Das AK, Alghamdi S, Soni K. Therapeutic application of microsponges based drug delivery systems. Curr Pharm Des. 2022;28(8):595-608. doi: 10.2174/1381612828666220118121536, PMID 35040411.
Gangadharappa HV, Gupta NV, Prasad M SC, Shivakumar HG. Current trends in microsponge drug delivery system. Curr Drug Deliv. 2013;10(4):453-65. doi: 10.2174/1567201811310040010, PMID 22974222.
Srivastava R, Pathak K. Microsponges: a futuristic approach for oral drug delivery. Expert Opin Drug Deliv. 2012;9(7):863-78. doi: 10.1517/17425247.2012.693072.
Rahman M, Almalki WH, Panda SK, Das AK, Alghamdi S, Soni K. Therapeutic application of microsponges based drug delivery systems. Curr Pharm Des. 2022;28(8):595-608. doi: 10.2174/1381612828666220118121536, PMID 35040411.
Li M, Gan J, Xu X, Zhang S, Li Y, Bian L. Preparation characterisation and in vitro anti-inflammatory activity of baicalin microsponges. Heliyon. 2024 Apr 4;10(7):e29151. doi: 10.1016/j.heliyon.2024.e29151, PMID 38617936.
Shinkar DM, Bhamare BS, Saudagar RB. Microsponges. Asian J Res Pharm Sci. 2016;6(2):77-84. doi: 10.5958/2231-5659.2016.00011.4.
Singh Rawat P, Dhyani A, Singh V, Juyal D. A brief review of micro sponges: an update. The Pharma Innovation Journal. 2017;6(5):134-9.
Chen G, Ushida T, T Tateishi. Poly(DL-lactic-co-glycolic acid) sponge hybridized with collagen micro sponges and deposited apatite particulates. J Biomed Mater Res. 2001;57(1):8-14. doi: 10.1002/1097-4636(200110)57:1<8::aid-jbm1135>3.0, PMID 11416843.
Shreya G, Velhal AB, Jadhav PD, Redasani VK. An updated review on formulation and evaluation of microsponge. Bol Environ Pharmacol Life Sci. 2023 Jan;12(2):31-9.
Raut AV, Kathar N, Sanap G. Micro sponges: the drug delivery system. Int J Pharm Sci. 2024;2(1):328341. doi: 10.5281/zenodo.10523750.
Sharma S, Sharma A, Kaur C. Microsponges: as a topical drug delivery system. Int J Pharm Sci Res. 2020;11(2):524-34. doi: 10.13040/IJPSR.0975-8232.11(2).524-34.
Kumari P, Mishra S. A comprehensive review on novel microsponges drug delivery approach. Asian J Pharm Clin Res. 2016;9(1):25-30.
Choudhary A, Akhtar MS. Microsponge drug delivery system: emerging technique in novel drug delivery system and recent advances. Res J Pharm Technol. 2022;15(10):4835-40. doi: 10.52711/0974-360X.2022.00812.
Tushir R, Dhall MD, Goel N. Preparation and in vitro evaluation of anti-fungal activity of clotrimazole loaded microsponge based gel. Scopus Indexed. 2025;18(1):7808-20. doi: 10.37285/ijpsn.2025.18.1.8.