
Int J Pharm Pharm Sci, Vol 17, Issue 8, 64-68Original Article
DESIGN AND EVALUATION OF ASHWAGANDHA CHITOSAN NANOPARTICLES FOR ENHANCED NEUROPROTECTION IN HUNTINGTON’S DISEASE
R. M. AKILA*, M. K. NITHIN
Department of Pharmaceutics, College of Pharmacy, Sri Ramakrishna Institute of Paramedical and Sciences, Coimbatore, Tamil Nadu, India
*Corresponding author: R. M. Akila; *Email: akilakathiresan1973@gmail.com
Received: 09 Apr 2025, Revised and Accepted: 23 Jun 2025
ABSTRACT
Objective: To develop and evaluate Ashwagandha-loaded nanoparticles for targeted therapy in Huntington’s disease (HD), aiming to enhance neuroprotection and prevent brain atrophy caused by synthetic antipsychotic drugs.
Methods: Molecular docking studies were initially conducted using a variety of phytoconstituents of Ashwagandha extract against the mutant huntingtin (mHTT) protein. Ashwagandha-loaded chitosan nanoparticles have been developed using the ionic-gelation method. Subsequently, a pre-formulation study was conducted to evaluate drug-excipient compatibility using Fourier-transform infrared spectroscopy (FTIR). Upon confirming compatibility, Ashwagandha-loaded chitosan nanoparticles were formulated using the ionic gelation method. Among the formulations, F1 was selected based on in vitro release at pH 7.4. The optimized formulation (F1) was further characterized for particle size, zeta potential and entrapment efficiency. Drug release kinetics were also studied to understand the release mechanism.
Results: Molecular docking studies revealed that Withaferin A, a key bioactive compound of Ashwagandha exhibited a binding affinity comparable to that of tetrabenazine against the mutant huntingtin (mHTT) protein. FTIR analysis confirmed compatibility between the drug and excipients. The nano formulation F1 showed controlled drug release (76% at 6 h) in pH 7.4, making it suitable for brain-targeted delivery, and showed small particle size between 62.3 nm and 86.5 nm and entrapment efficiency (75.06%), indicating effective drug encapsulation in nanoparticulate form, potentially improving solubility and bioavailability. The kinetic studies revealed that the formulation F1 followed zero-order kinetics and provide evidence to the diffusion mechanism of drug release.
Conclusion: The Ashwagandha-loaded chitosan-based biodegradable nano formulation may serve as a suitable alternative to conventional synthetic antipsychotic drugs for Huntington’s disease by demonstrating comparable efficacy in targeting mutant huntingtin protein. It enhances brain-targeted neurotrophic delivery, promotes neurogenesis, and prevents brain atrophy in HD. However, further in vivo validation is required.
Keywords: Ashwagandha, Chitosan nanoparticles, Mutant huntingtin protein, Molecular docking, In vitro release kinetics
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijpps.2025v17i8.54517 Journal homepage: https://innovareacademics.in/journals/index.php/ijpps
INTRODUCTION
Huntington’s disease – also known as Huntington’s chorea – is an incurable, progressive degenerative brain disorder caused by a genetic mutation leading to abnormal (mHTT) protein aggregation, neuronal dysfunction, motor, cognitive and psychiatric impairments. Current treatments primarily focus on symptom management and antipsychotic drugs are commonly prescribed to manage symptoms such as chorea and behavioral changes; they come with significant drawbacks. Long-term use of these drugs has been associated with brain shrinkage, dopamine dysregulation, and worsened cognitive decline, further accelerating neuronal degeneration in HD patients [1].
Given these limitations, there is a growing interest in natural neuroprotective agents that can slow disease progression without causing additional brain atrophy. Ashwagandha (Withania somnifera), a well-documented adaptogenic herb in Ayurvedic medicine, has shown promising effects in reducing neuroinflammation [2], enhancing neuronal survival, and even reversing brain shrinkage. Preclinical studies suggest that its active compounds, withanolides, can promote neurogenesis, protect against oxidative stress [3] and modulate neurotransmitter function, key mechanisms that could offer a safer alternative or complementary approach to HD management.
This research work focuses on the nano formulation strategies for delivering Ashwagandha effectively in Huntington’s disease treatment, ensuring enhanced bioavailability and targeted neuroprotection to counteract brain atrophy and improve patient outcomes.
MATERIALS AND METHODS
Ashwagandha root extract (M/s Natural Remedies Pvt Ltd., Bengaluru, India), Chitosan Sigma Aldrich, Mumbai, India, Sodium Tri-polyphosphate Sigma Aldrich, Mumbai, India, Acetic acid Sigma Aldrich, Mumbai, India, Sodium hydroxide Sigma Aldrich, Mumbai, India, Sodium chloride Hi media labs Ltd, Bangalore, Ethanol Qualigens fine chemicals, Bangalore. Autodock software 4.2.
Docking study of mutant huntingtin protein (m HTT)
Macromolecular structure databases, small molecule repositories, molecular docking tools are integral components of in silico drug discovery. The three-dimensional structure of the mutant huntingtin (mHTT) protein was retrieved from the RCSB Protein Data Bank in. pdb format. Following that, the AutoDock Tools 4.2 was used to prepare and visualize the mutant huntingtin (mHTT) receptor. First, the target protein was isolated from the crystal structure by removing any extraneous ligand. Next, crystallographic water molecules were removed, as they may interfere with docking accuracy and binding affinity calculations. Hydrogen atoms were then added to the protein structure to ensure proper bonding geometry. Finally, the processed structure was saved in. pdb format for docking studies.
The molecular structures of the ligands Withaferin A, Sitoindoside IX, Physagulin D, Withanoside IV, and Viscosalactone-B were retrieved from PubChem (https://pubchem.ncbi.nlm.nih.gov/) in SDF format and converted to PDB format using standard cheminformatics tools. The target protein and phytoconstituent ligands from Ashwagandha were subsequently prepared in PDBQT format for molecular docking using AutoDock WS. During preparation, nonpolar hydrogen atoms were merged, and Gasteiger partial charges were assigned to the protein, which was maintained in a rigid conformation. Torsional flexibility was applied to all rotatable bonds in the ligands.
Molecular docking was performed using AutoDock WS, with the ligands docked to the active site of mutant huntingtin (mHTT). The grid box dimensions were set to 72 Å × 36 Å × 84 Å, centered at coordinates x = 10.147, y = −29.237, z = −52.564. These grid parameters were defined based on the active site residues of mHTT, identified from high-resolution X-ray crystallography data [4].
Pre-formulation study
Drug-excipient compatibility study
FTIR analysis was performed to study the chemical interaction between drug and polymer using JASCO FTIR 4100 as a pre-formulation study. The samples were scanned in the IR range from 400 to 4000 cm−1 [5].
Formulation of ashwagandha nanosuspension
Ashwagandha root extract was encapsulated in chitosan nanoparticles using an ionic gelation method. 50 mg of chitosan was dissolved in 5 ml of 1% glacial acetic acid using a magnetic stirrer at 1000 rpm for one hour to produce the chitosan solution. Ashwagandha was dissolved in a 2:8 mixture of ethanol and phosphate buffer with a pH of 7.4 to form the Ashwagandha solution. The above drug solution was combined with 85 ml of 0.5% v/v Tween80 solution before being added to the chitosan solution. By adding 1N NaOH, the pH of the solution was brought down to 4.6-4.8. As a cross-linking agent, 10 ml of STPP (sodium tripolyphosphate) was then added drop wise to the above solution under stirring at 1000 rpm for 20 min on a magnetic stirrer, and sonicated for 30 min [5] (table 1).
Table 1: Formula for Ashwagandha-loaded chitosan nanosuspension
| S. No. | Formulation code | Drug (mg) | Chitosan (mg) | Tween 80 (%v/v) | STPP (ml) |
| 1 | F1 | 25 mg | 25 mg | 1.5 | 10 |
| 2 | F2 | 25 mg | 50 mg | 0.5 | 10 |
| 3 | F3 | 25 mg | 75 mg | 1 | 10 |
Evaluation of ashwagandha-loaded chitosan nanosuspension
Morphological examination
TEM (JEOL-JEM1010, Japan) was used to examine the actual particle size and shape of optimised Ashwagandha nanosuspensions. A drop of the diluted nanosuspension was applied to a copper grid with carbon coating before being stained with a drop of phospho-tungstic acid, 1% w/w. Samples were stained and then examined with a TEM [6].
In vitro drug release study of the prepared formulations
Using a magnetic stirrer and a dialysis membrane, the in vitro drug release study of the optimized batch was carried out. A dialysis bag was soaked in distilled water an hour before usage, and the bag was connected to the device's paddle. The drug-loaded nanoparticles were redispersed in 2 ml of phosphate buffer (pH 7.4). The dissolution medium, 500 ml of phosphate buffer of pH 7.4 kept at 37 °C ±1 °C, was mixed using a magnetic stirrer that was rotated at 100 rpm. At predetermined intervals, 5 ml of the sample aliquots were taken and replaced with an equivalent volume of fresh dissolution medium. After spectrophotometric analysis of the samples at λmax = 216 nm against an appropriate blank, the amount of drug released at different time intervals was estimated. The results are expressed in terms of the average percentage drug release±standard deviation [7].
Measurement of mean particle size
The mean particle size of the Ashwagandha-loaded chitosan nanosuspension was determined using the particle size analyzer (Malvern version 7.13). In order to assess the vesicle diameter, 100 μl of the formulation were diluted in a 2:8 ratio with an adequate volume of ethanol and PBS pH 7.4 before being used [8].
Measurement of zeta potential
The (Malvern Zetasizer) was used to test the zeta potential of the optimised phytosome solution. In order to evaluate the zeta potential, 1 ml of the sample was diluted with water to make 10 ml, and 5 ml of this diluted sample was then transferred to a cuvette [9].
Determination of entrapment efficiency
The entrapment efficiency was determined by measuring the concentration of the free drug in the dispersion by ultra-centrifugation of samples at 10,000 rpm for 30 min. The amount of free ashwagandha was determined in clear supernatant by UV spectrophotometry at 216 nm using supernatant of non-loaded (blank) nanoparticles as basic correction and calculated using the following equation [10].

The kinetics of drug release from chitosan nanoparticles loaded with ginger were calculated using an Add-In for Microsoft Office Excel. The acquired formulation's in vitro drug dissolution data were fitted to zero-order, first-order, and the Higuchi model (cumulative percentage of drug release versus log time) in order to assess the kinetic modeling of drug release. The best-fit model was defined as the one with a greater correlation coefficient, or higher R2 [11].
RESULTS AND DISCUSSION
Molecular docking study of mutant huntingtin protein (m HTT)
The selected standard drug tetrabenazine significantly interacted with selected protein targets of HD. The binding energy was observed in the range of − 4.711 to −6.903 and it was compared with the test drug Withania somnifera, containing Withaferin-A (-6.11), Sitoindoside-IX (-4.34), Physagulin-D (-5.24), Withanoside-IV (-1.3), Viscosalactone-B (-5.4) has a good docking active binding energy against the mutant huntingtin protein.
Drug-excipient compatibility study
Using the FTIR peak matching approach, the compatibility of Ashwagandha and chitosan was assessed. The lack of any peak appearance or disappearance in the drug-polymer mixture demonstrated that there was no chemical interaction between the drug and polymer (fig. 1).
Formulation of ashwagandha nanosuspension
In this ionic gelation method of preparation of nanoparticles, chitosan in an acetic-acid solution acts as a source of the cation; when added to a polyanionic sodium tripolyphosphate solution, complexation occurs due to electrostatic attraction between oppositely charged species; chitosan undergoes ionic gelation and precipitates to form spherical particles. Tween 80 is a surfactant adsorbed onto the surface of nanoparticles that prevents agglomeration, reduces surface tension, and thereby stabilizes the colloidal system. Comparatively with the Ashwagandha nano formulation of Madhu et al. (2021) [12] by solvent-evaporation technique with poly-lactic-co-glycolic acid (PLGA), this method provides a solvent-free, environmentally friendly, mucoadhesive, and biocompatible alternative.
Evaluation of ashwagandha-loaded chitosan nanosuspension
Morphological examination
Using transmission electron microscopy (TEM), the size and shape of the particles were investigated. The optimized chitosan nanosuspension (F1) with drug loading showed up as segregated, uniformly sized, and spherically shaped in the transmission electron microscopy image. The range of nanoparticle sizes was 62.3 nm to 86.5 nm. The objectives of drug delivery strategies mediated by nanoparticles are in line with these features, which promote improved pharmacokinetic characteristics and therapeutic efficacy.
In vitro drug release study of the prepared formulations
The in vitro release profile of Ashwagandha chitosan-loaded nanoparticles for different formulations is shown. The percentage of drug release was not the same for all the formulations; it varied with time, and it was found that formulation F1 had the maximum percentage release of 76.4±2.63% at the end of the 6th hour (fig. 3), indicating a controlled and sustained release behaviour of the Ashwagandha-loaded chitosan nanoparticles due to the polymer matrix formed by chitosan and STPP.
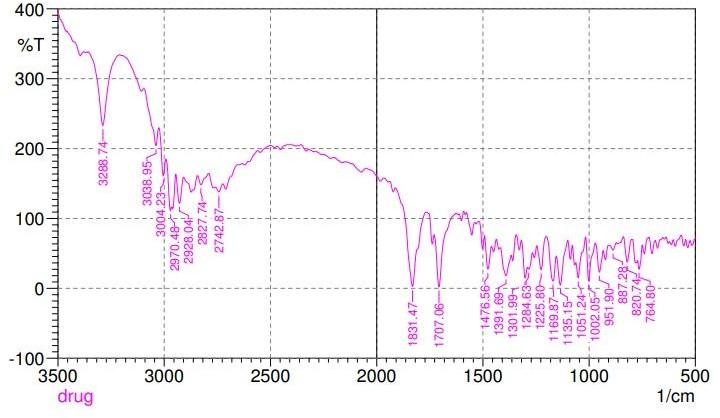
Fig. 1: Drug-excipient compatibility study

Fig. 2: TEM image of Ashwagandha-loaded chitosan nanosuspension of optimized formulation (F1)
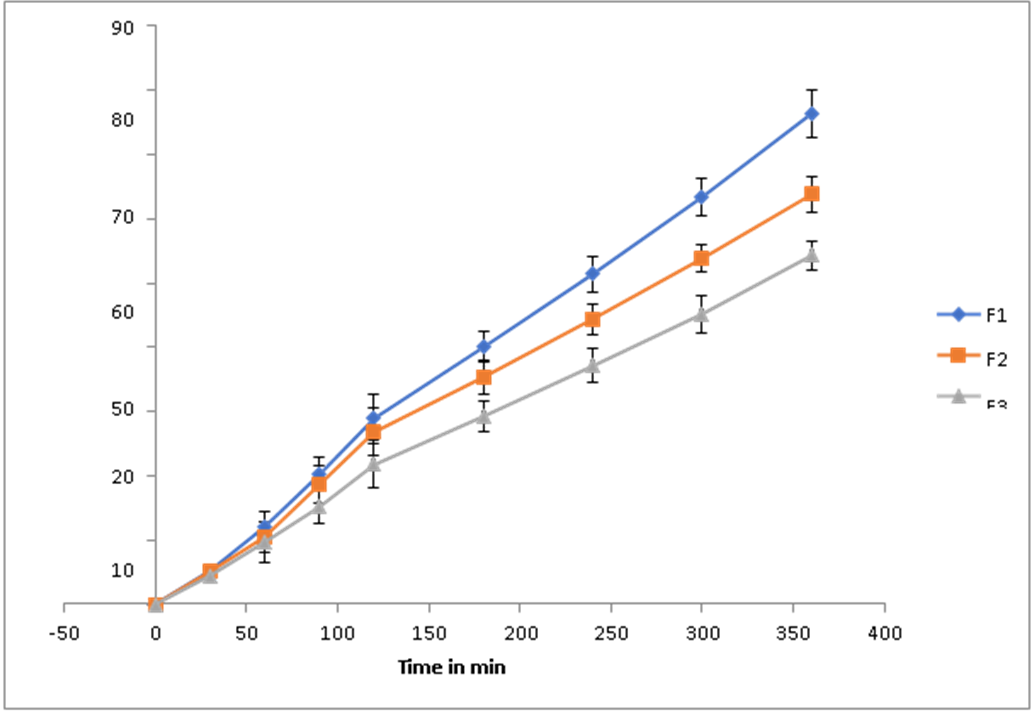
Fig. 3: In vitro release of Ashwagandha loaded chitosan nanosuspension of formulations F1, F2 and F3, datas are represented as mean ±SD (n=3)
Measurement of particle size
Particle size analyzer (Malvern version 7.13) was used to measure the mean size of chitosan nanoparticles loaded with Ashwagandha. According to the fig. 4, the drug-loaded chitosan nanoparticles had an average particle size of 112.9 nm, indicating favorable size range for nanoparticles used in drug delivery. This size range facilitates enhanced solubility, absorption and cellular uptake by endocytosis, making the formulation promising for targeted and controlled release of Ashwagandha [13].
Measurement of zeta potential
The most crucial factor influencing the physical stability of Ashwagandha-loaded chitosan nanoparticles is their zeta potential. The stability increases with increasing electrostatic repulsion between the particles. Good physical stability of the dispersion is predicted by a zeta potential value more than+20 mV or less than-20 mV [14]. Zeta potential of the optimized formulation F1 was found to be 25.8mV and was moderately stable, indicating particles have enough surface charge to repel each other and stay dispersed for a reasonable time.
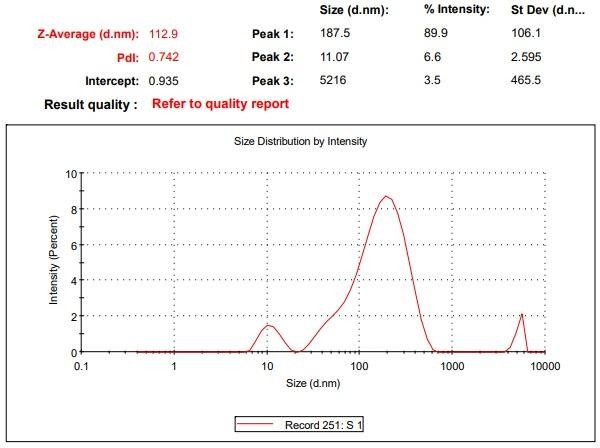
Fig. 4: Particle size distribution of formulation F1
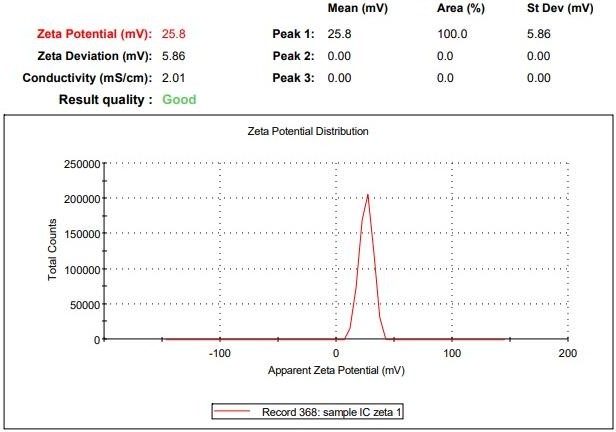
Fig. 5: Zeta potential of ashwagandha-loaded chitosan nano-formulation
Determination of ashwagandha entrapment efficiency
The entrapment efficiency of drugs depends on polymer concentration. As the polymer concentration increases, % entrapment efficiency increases, [15] and ranges between 75.06% and 87.2 % suggests good reproducibility can potentially enhance the bioavailability of Ashwagandha by protecting its active compounds and improving their controlled release (table 2).
Table 2: Percentage entrapment efficiency
| S. No. | Formulation code | Entrapment efficiency (EE %) |
| 1 | F1 | 75.06±0.03 |
| 2 | F2 | 77.50±0.65 |
| 3 | F3 | 87.20±1.23 |
Data are expressed as mean±SD (n=3)
In vitro drug release kinetics
To analyse the release mechanism and release rate kinetics of the dosage form, the data obtained from in vitro drug release from optimized formulation were fitted to model’s representation zero order, first order, Higuchi and Korsmeyer-Peppas. The data indicates a high linearity when plotted by the zero-order equation. Hence, it can be concluded that the major mechanism of drug release follows zero-order kinetics. Data based on the zero-order models usually provide evidence to the diffusion mechanism of drug release from controlled release delivery system (table 3) [16].
Table 3: In vitro drug release kinetics
| Formulation code | R2 value | |||
| Zero order | First order | Higuchi | Korsemeyer-peppas | |
| F1 | 0.997 | 0.994 | 0.936 | 0.681 |
| F2 | 0.997 | 0.994 | 0.936 | 0.681 |
| F3 | 0.997 | 0.994 | 0.936 | 0.681 |
CONCLUSION
Ashwagandha-loaded chitosan nanoparticles were successfully formulated to enhance its neuroprotective potential for Huntington’s disease, offering a natural alternative to synthetic drugs, which can cause severe side effects. The controlled release of the formulation, enhanced bioavailability, successful molecular docking, and advantageous pharmacokinetics indicate that these nanoparticles represent a promising therapeutic option. Further in vivo studies in animals and humans are needed to validate these findings.
ACKNOWLEDGEMENT
The authors sincerely thank Dr M. Gopal Rao, Professor and Head, Department of Pharmaceutics, College of Pharmacy, Sri Ramakrishna Institute of Paramedical Sciences, Coimbatore, which is affiliated with The Tamilnadu Dr. M. G. R. Medical University, Chennai, for providing the necessary facilities to conduct this research.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
RM Akila conceptualized the study, designed the research, analyzed the data and drafted the manuscript. MK Nithin contributed in assisting data collection and preliminary analysis under supervision. Both authors reviewed and approved the final version of the manuscript.
CONFLICT OF INTERESTS
The authors declare that there is no conflict of interests regarding the publication of this paper.
REFERENCES
Ho BC, Andreasen NC, Ziebell S, Pierson R, Magnotta V. Long term antipsychotic treatment and brain volumes: a longitudinal study of first episode schizophrenia. Arch Gen Psychiatry. 2011 Feb;68(2):128-37. doi: 10.1001/archgenpsychiatry.2010.199, PMID 21300943.
Krishna Raju AV, Somepalli V, Thanawala S, Shah R. Efficacy and anti-inflammatory activity of ashwagandha sustained release formulation on depression and anxiety induced by chronic unpredictable stress: in vivo and in vitro studies. J Exp Pharmacol. 2023 Jul 25;15:291-305. doi: 10.2147/JEP.S407906, PMID 37521489.
Cui X, Lin Q, Liang Y. Plant-derived antioxidants protect the nervous system from aging by inhibiting oxidative stress. Front Aging Neurosci. 2020 Jul;12(12):209. doi: 10.3389/fnagi.2020.00209, PMID 32760268.
Deshpande SH, Muhsinah AB, Bagewadi ZK, Ankad GM, Mahnashi MH, Yaraguppi DA. In silico study on the interactions, molecular docking dynamics and simulation of potential compounds from Withania somnifera (L.) dunal root against cancer by targeting KAT6A. Molecules. 2023 Jan 22;28(3):1117. doi: 10.3390/molecules28031117, PMID 36770785.
Akila RM, Maria Shaji D. Ginger loaded chitosan nanoparticles for the management of 3–nitropropionic acid induced huntingtons disease like symptoms in male Wistar rats. Int J Pharm Pharm Sci. 2022 Jan 1;14(1):28-36. doi: 10.22159/ijpps.2022v14i1.42894.
Md S, Alhakamy NA, Akhter S, Awan ZAY, Aldawsari HM, Alharbi WS. Development of polymer and surfactant-based naringenin nanosuspension for improvement of stability antioxidant and antitumour activity. J Chem. 2020 Jul 15;2020(2):1-10. doi: 10.1155/2020/3489393.
Remya PN, Damodharan N. Formulation development and characterization of nimodipine loaded solid lipid nanoparticles. Int J Appl Pharm. 2020;12(5):265-71. doi: 10.22159/ijap.2020v12i5.34342.
Patel PN, Patel LJ, Patel JK. Development and testing of novel tamoxifen citrate-loaded chitosan nanoparticles using ionic gelation method. Pharm Sin. 2011;2(4):17-25.
Ramaye Y, Dabrio M, Roebben G, Kestens V. Development and validation of optical methods for zeta potential determination of silica and polystyrene particles in aqueous suspensions. Materials (Basel). 2021;14(2):290. doi: 10.3390/ma14020290, PMID 33429974.
Baby AA, Sree N, Harsha K, Jayaveera N. Formulation and evaluation of levofloxacin nanoparticles by ionic gelation method. J Pharm Pharm Sci. 2012;1(1):7-15.
Patil Jayashri, Patil R. Formulation and evaluation of besifloxacin non-erodible ocular inserts. Int J Appl Pharm. 2022 Jan 7;14(1):148-55. doi: 10.22159/ijap.2022v14i1.43058.
Madhu S, Komala M, Pandian P. Formulation development and characterization of withaferin a loaded polymeric nanoparticles for alzheimers disease. Bio Nano Sci. 2021;11(2):559-66. doi: 10.1007/s12668-020-00819-w.
Foroozandeh P, Aziz AA. Insight into cellular uptake and intracellular trafficking of nanoparticles. Nanoscale Res Lett. 2018 Oct 25;13(1):339. doi: 10.1186/s11671-018-2728-6, PMID 30361809.
Bhattacharjee S. DLS and zeta potential: what they are and what they are not? J Control Release. 2016 Aug 10;235:337-51. doi: 10.1016/j.jconrel.2016.06.017, PMID 27297779.
Robbani S, Elya B, Iswandana R. Alpha glucosidase and dpp-iv inhibitory activities of ethanol extract from caesalpinia sappan andrographis paniculata and syzygium cumini. Pharmacogn J. 2022;14(3):702-9. doi: 10.5530/pj.2022.14.89.
Costa P, Sousa Lobo JM. Modeling and comparison of dissolution profiles. Eur J Pharm Sci. 2001 May;13(2):123-33. doi: 10.1016/s0928-0987(01)00095-1, PMID 11297896.