
Int J Pharm Pharm Sci, Vol 17, Issue 9, 36-46Original Article
EXTRACTION AND ISOLATION OF BIOACTIVE COMPOUNDS FROM CORAL JUNCEELLA DELICATA (GRASSHOFF, 1999) FROM WEST COAST OF MUMBAI
MEENAKSHI BORATE, GAUTAM ZODAPE*
Department of Zoology, Chikitsak Samuha’s Sir Sitaram and Lady Shantabai Patkar College of Arts and Science and V. P. Varde College of Commerce and Economics, S. V. Road, Goregaon West, Mumbai-400104, Maharashtra, India
*Corresponding author: Gautam Zodape; *Email: drgautamvz5@gmail.com
Received: 16 Apr 2025, Revised and Accepted: 07 Jul 2025
ABSTRACT
Objective: To isolate the bioactive compounds from coral Junceella delicata (Grasshoff, 1999) collected from West coast of Mumbai.
Methods: The coral J. delicata was collected from Patwadi Village, Madh Island, Malad West Mumbai-400061, Maharashtra, India, during the low tide. The sample was grinded with blender and then macerated by adding an equal volume of MeOH: DCM (1:1). The aliquot was concentrated in a rotary vacuum evaporator at 45 °C. The resultant compound was subjected to Millipore filter system and finally dried in vacuum desiccator. The sample was further subjected for TLC and pure compounds were processed for GC-MS and FTIR for structural determinations.
Results: The distinct and well-separated compounds were processed for GC-MS and FTIR for their structural elucidation. These compounds are (Ethyl aminomethyl formimidate); (Gly-Gly); (2-(2-Pyridyl)-4 methylthiazole-5-carboxylic acid); (7-Methoxy-2-methylquinolin-4-ol); (Fraxidin); (2-methyl-3-trans-propenylpyrazine); (3-tert-Butylpyridine); (Acetaldehyde benzyl ethyl acetal); (α-Methylcinnamic acid); (4-Ethoxycoumarin); (3-Hydroxycoumarin); (2, 4, 7, 9-Tetramethyl-5-decyne-4, 7-diol); (2,2-Bis(3-allyl-4-hydroxyphenyl) propane); (Phenyltriethylammonium cation); (Dodecanedioic acid). These compounds showed biomedical properties.
Conclusion: The isolated bioactive compounds may be used for pharmaceutical and therapeutic applications. Further studies, including molecular-level research, are necessary to confirm their mechanisms of action and clinical relevance. Additionally, safety and efficacy assessments are crucial to support the development of new pharmaceutical products aimed at improving human health.
Keywords: Coral, Madh island, Extraction, Bioactive compounds, Analytical techniques, Structural determination
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijpps.2025v17i9.54605 Journal homepage: https://innovareacademics.in/journals/index.php/ijpps
INTRODUCTION
Marine organisms are being studied for their bioactive potential. Many invertebrates, including corals, have yielded a vast amount of extremely potent anti-tumour compounds. Coral reefs are the biggest ecosystems in the oceans. Soft corals play a crucial role in creating lead compounds for drugs through their various defense mechanisms: they protect their living space from competitors, combat pathogenic microorganisms with toxic metabolites, and release these substances to survive [1, 2]. Soft corals belong to the Cnidaria phylum, class Anthozoa, subclass Octocorallia, and order Alcyonacea, consist of various genera within the families Xeniidae, Nephtheidae, and Alcyoniidae [3]. Soft corals are present in all parts of the ocean, ranging from warm shallow waters to cold deep waters [4]. Soft corals, unlike hard corals in the Anthozoa class, lack the protective calcium carbonate skeleton found in hard corals. Hence, soft corals employ toxic and bioactive substances for defense against predators [5, 6]. Research conducted on soft corals demonstrated that substances released by them result in the demise of hard corals in their vicinity [7]. Several marine natural compounds have been extracted from soft corals. For instance, research on cytotoxic and anti-viral activities of marine organisms in the Red Sea indicated that soft corals Sarcophyton trochliophorum and Litophyton arboreum exhibit strong activity against HeLa and U-937 cancer cell lines. These soft corals contain new bioactive compounds that may have cytotoxic, anti-inflammatory, HIV-inhibiting, antibacterial, anti-tumour, and antifungal properties. According to [8], soft corals contain a variety of rich biomedical compounds such as steroids, alkaloids, terpenoids, prostaglandins, sterols, and steroid glycosides. Research has explored the antimicrobial properties of soft corals from the genera Parerythropodium, Dendronephthya, Lobophytum, and Sarcophyton [9-12]. Cnidarian toxins are the subject of extensive research in Japan, China and some Western countries. Between 1969 and 2016, there was a decrease in new U. S. Food and Drug Administration (FDA) approvals, with the highest number of approvals in 1996 (53 new molecular entities (NMEs)/y) and the lowest in 2010 and 2016 (15 NMEs/y) [13]. Since soft corals is a promising and underexplored source of bioactive compounds with significant pharmaceutical potential. Further research into their chemical diversity could lead to the discovery of novel therapeutic agents, especially in the field of cancer treatment.
MATERIALS AND METHODS
Sample collection
The coral J. delicata was collected from Patwadi Village, Madh Island, Malad West Mumbai-400061, Maharashtra, India (19⁰ 8’ 47652” N, 72⁰ 47’ 17.8116” E) during the low tide. The debris was removed during collection. The sample collected was washed twice with sea water and then rinse three times with distilled water and stored in ice cubes until they were transferred to the deep freezer at 8⁰ C at the Department of Zoology, S. S. and L. S. Patkar College of Arts and Science, and V. P. Varde College of Commerce and Economics, Goregaon West, Mumbai-400104.
Identification of coral
Preliminary identification was done by examining the shape and size of the sclerites and by reviewing the literature. The confirmation of identification was done by Dr. Swapnaja Mohite, Professor and Head, Department of Fisheries Biology, College of Fisheries, Shirgaon, Ratnagiri, Maharashtra-415629.
Preparation of crude extract
The coral sample was removed from the deep fridge and bloat with blotting paper and kept in shed dried for 48 h. After 48 h the sample was grinded with blender and then macerated by adding an equal volume of MeOH: DCM (1:1) for 24 h in the water bath at 45 °C. The aliquot mixture obtained was filtered through Whatman filter paper 1. The homogenate was centrifuged at 10,000 rpm for 15 min in cold centrifuge (Remi centrifuge serial No. VCDX-5983) at -8 °C and supernatant was collected. The aliquot was concentrated in a rotary vacuum evaporator at 45 °C. The resultant compound was subjected to Millipore filter system and finally dried in vacuum desiccator and stored in the refrigerator at-20 °C till further use.
Ethical approval
Ethical approval was sought from the Principal Chief Conservator of Forest, Nagpur (Desk-22(8)/Res/CR-25(22-23)/1431/(22-23) and final approval was taken from the Maharashtra State Biodiversity Board, Nagpur (MSBB/Desk-5/825/2022-23) for collection of J. delicata samples. The voucher specimen of J. delicata was submitted to the repository at the Zoological Survey of India, Western Regional Office, Pune (ZSI-WRC Misc/18), India.
TLC analysis
CAMAG HPTLC model available at Anchrom test lab. Mulund, Mumbai, was used for the analysis of the samples. In this system stationary phase was precoated with an aluminum plate containing silica gel (60F254), whereas the mobile phase was a mixture of chloroform, toluene, and ethanol in a 4:4:1 (v/v/v) ratio. The development of the sample spots was done using twin trough chamber. The Deutorium lamp at 254 nm was used for densitometric scanning of the sample.
GC-MS: (GAS chromatography-mass spectrometry)
The samples were analyzed on GC-MS at the Sophisticated Analytical Instruments Facility (SAIF), IIT Madras, The Agilent Model 8890 GC System with Single Quadrupole Mass Spectrometer (5977B MSD) analyzer is used for the separation and identification of thermally stable volatile compounds. The GC consists of Split/Splitless (SSL) injectors and capillary columns for different applications. NIST spectral library search was used to identify the molecules.
FTIR-spectrophotometer
FTIR spectrophotometers installed at SAIF-IIT Powai, Mumbai were used for the characterization of the bioactive compounds. The model Bruker Hyperion 3000 Microscope connected to a Vertex 80 FTIR System were used. For FTIR, the samples were mixed in KBr and pallets were formed. The scanning was done in the range of 4000 cm-1 to 400 cm-1. All the chemicals and reagents used for IR analysis were of analytical grade. Analytical grade solvents and chemicals were used from M/S. S. D. Fine Chemicals, Thane, India.
RESULTS AND DISCUSSION
Characterization of crude extract by HPTLC
The extracts isolated from the coral J. delicata collected from Madh Island, Malad West Mumbai are spotted on HPTLC plates and the plates were developed in a twin-trough chamber using Chloroform: Toluene: Ethanol in a ratio of 4:4:1 (v/v/v). The plates were dried and sprayed with the anisaldehyde sulfuric acid reagent. The development of the sample spots was done using twin trough chamber. The deutorium lamp at 254 nm was used for densitometric scanning of the sample shown in fig. 1. The spraying reagents gave positive tests for the presence of compounds. The distinct and well-separated spots were at Rf values MG-0.38, MY-0.48 and MO-0.78 were taken for analysis, whereas the 0.07, 0.11, 0.18, 0.36, 0.39, and 0.70 were rejected because of overlapping to one another. The densitometric scanning of these spots resulted in the quantification of these substances and found to be 0.07(4.21%), 0.11(17.58%), 0.18(19.84%), 0.29 (5.14%), 0.38(17.93%), 0.48(12.44%), 0.70 (12.82%), and 0.78(10.05%) respectively shown in fig. No. 1. Preparative TLC was performed and the spots at Rf values at MG-0.38, MY-0.48 and MO-0.78 were isolated by scrapping the spots into methanol. Pure compounds were obtained by evaporating the solvent methanol. These compounds are then characterized by FTIR technique.
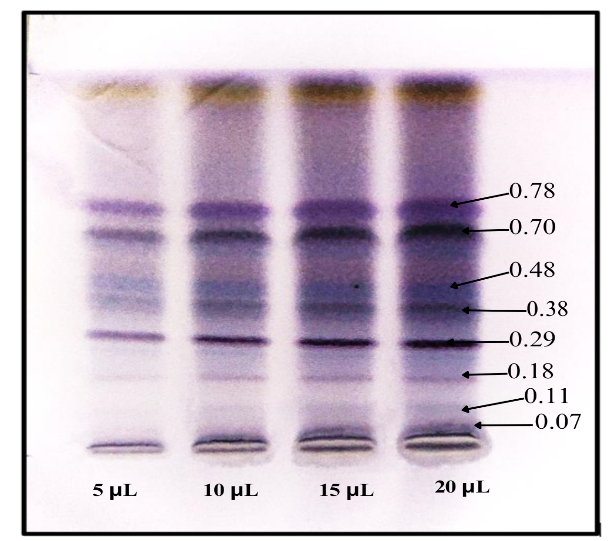
Fig. 1: Photograph showing the separation of bioactive compounds of crude extract of coral J. delicata by high-performance thin-layer chromatography
Characterization of isolated extracts of coral J. delicata by GC-MS
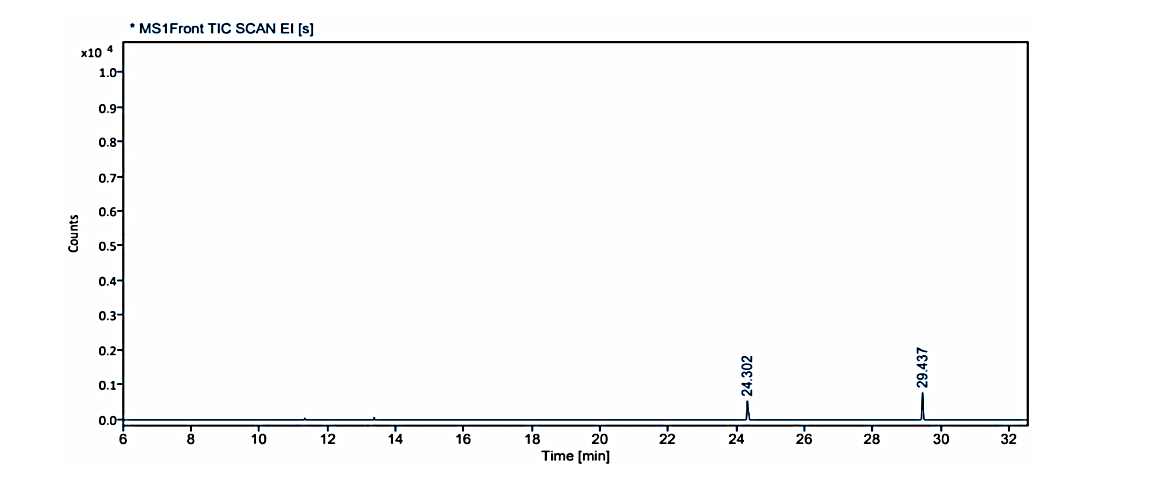
Fig. 1.1: Gas chromatogram showing the isolated compound no. 1 (MG) at Rf value -0.38 on TLC
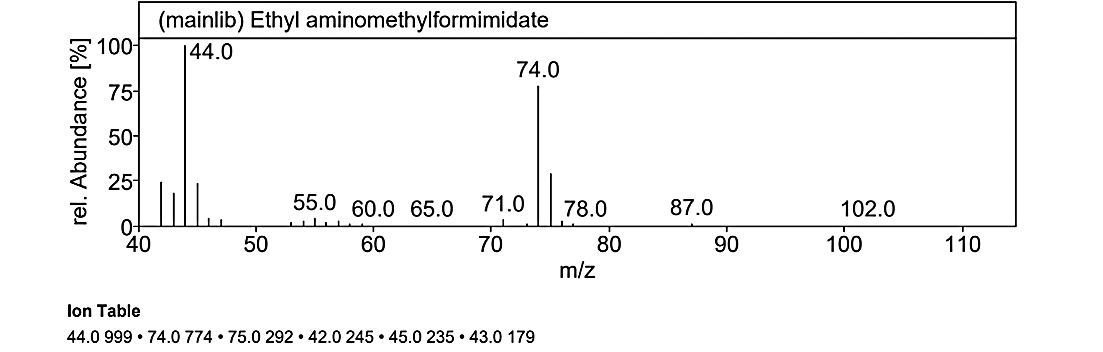
Fig. 1.1a: Mass spectra of the compound No.1 (MG) at RT value 29.437
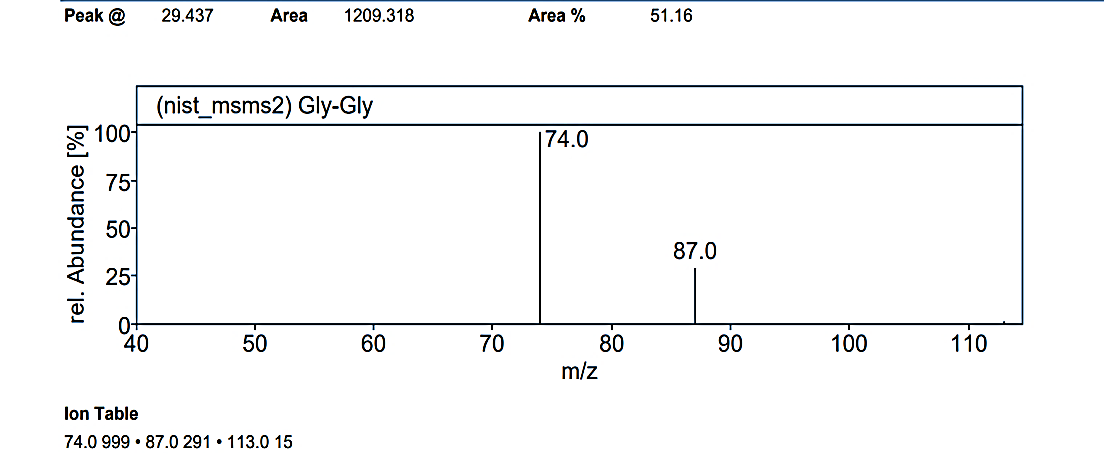
Fig. 1.1b: Mass spectra of the compound No.1 (MG) at RT value 29.437
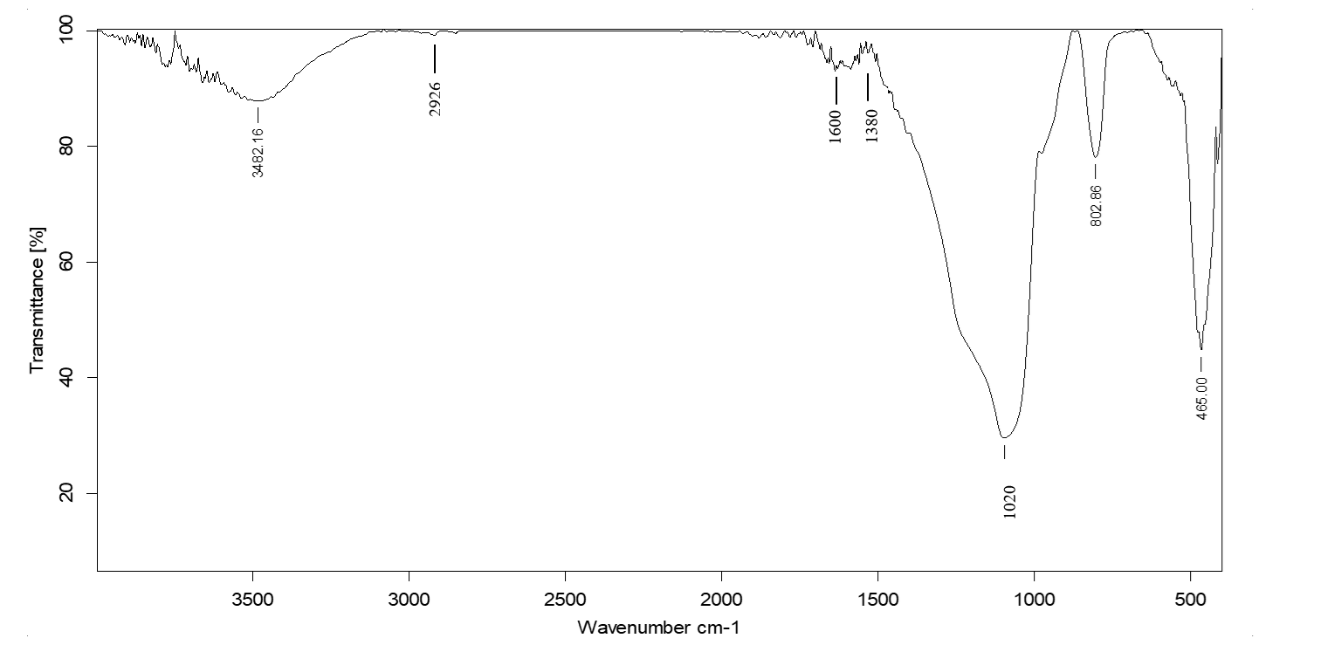
Fig. 2.1: FTIR spectra of the isolated compound No.1 (MG)-0.38 on TLC
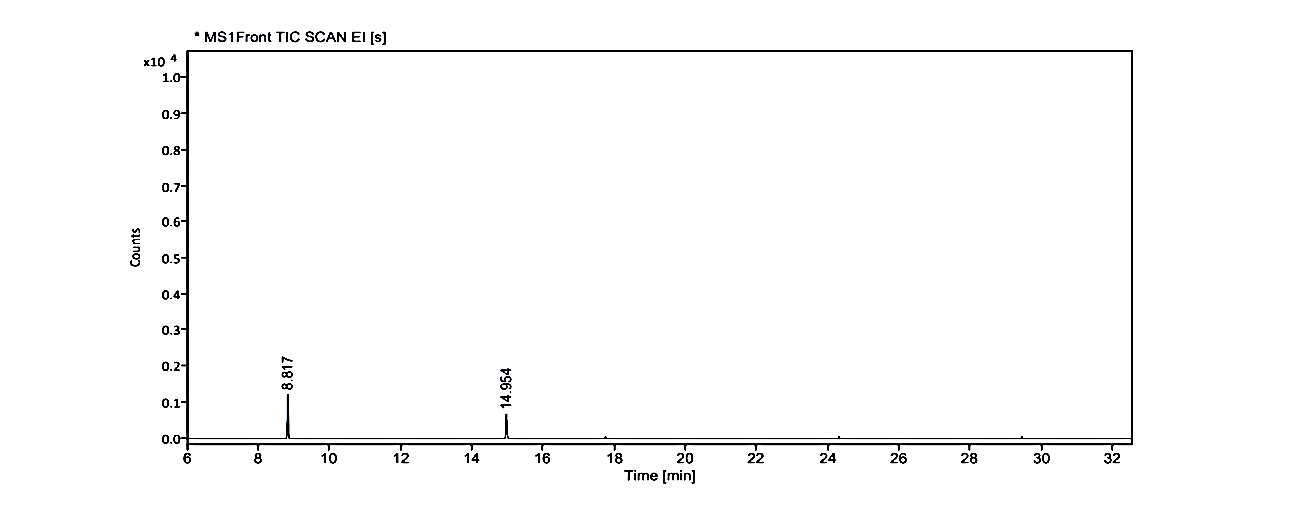
Fig. 1.2: Gas chromatogram showing the isolated compound No. 2 (MY) at Rf value -0.48 on TLC
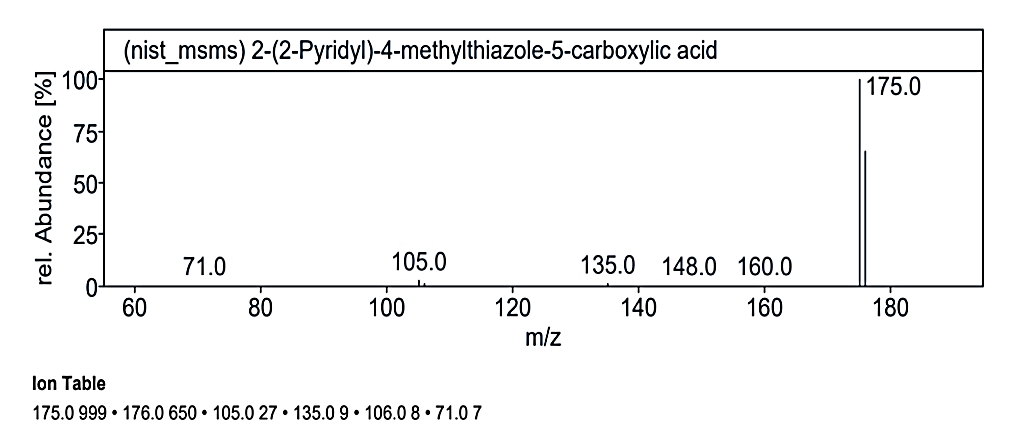
Fig. 1.2. a: Mass spectra of the compound No. 2 (MY) at RT value 8.817
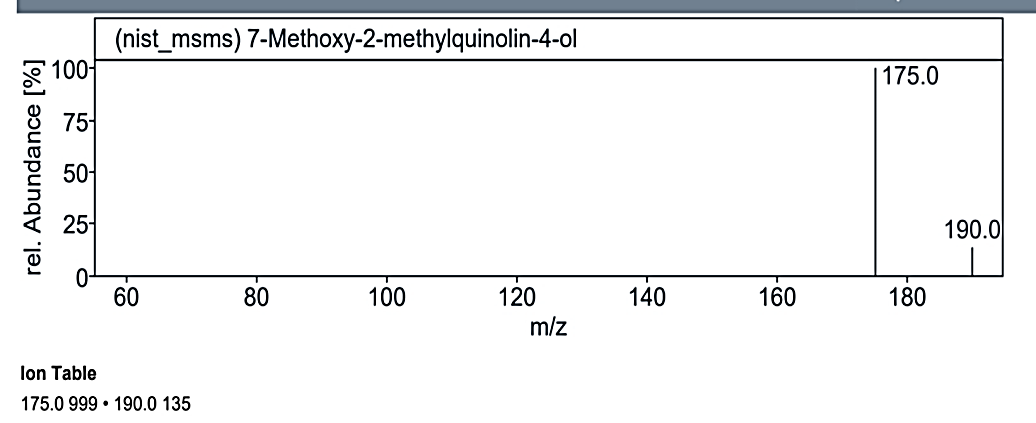
Fig. 1.2b: Mass spectra of the compound no. 2 (MY) at RT value 8.817
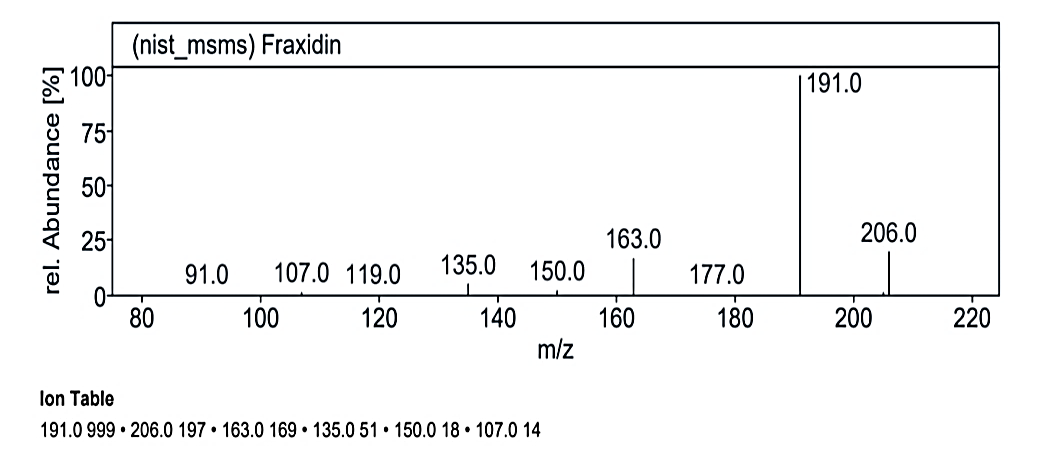
Fig. 1.2c: Mass spectra of the compound no. 2 (MY) at RT value 14.954
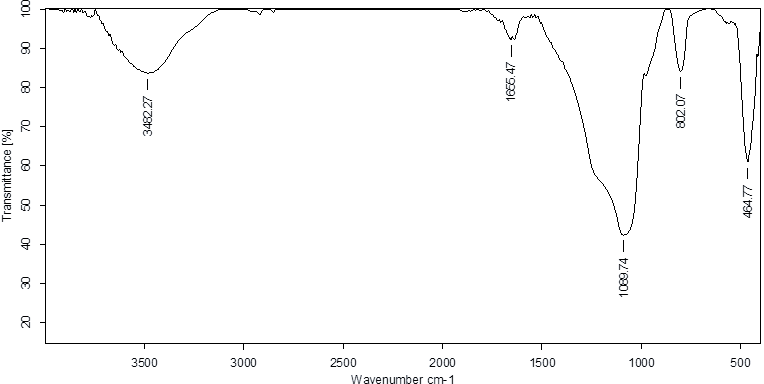
Fig. 2.2: FTIR spectra of the isolated compound No. 2 (MY)-0.48 on TLC
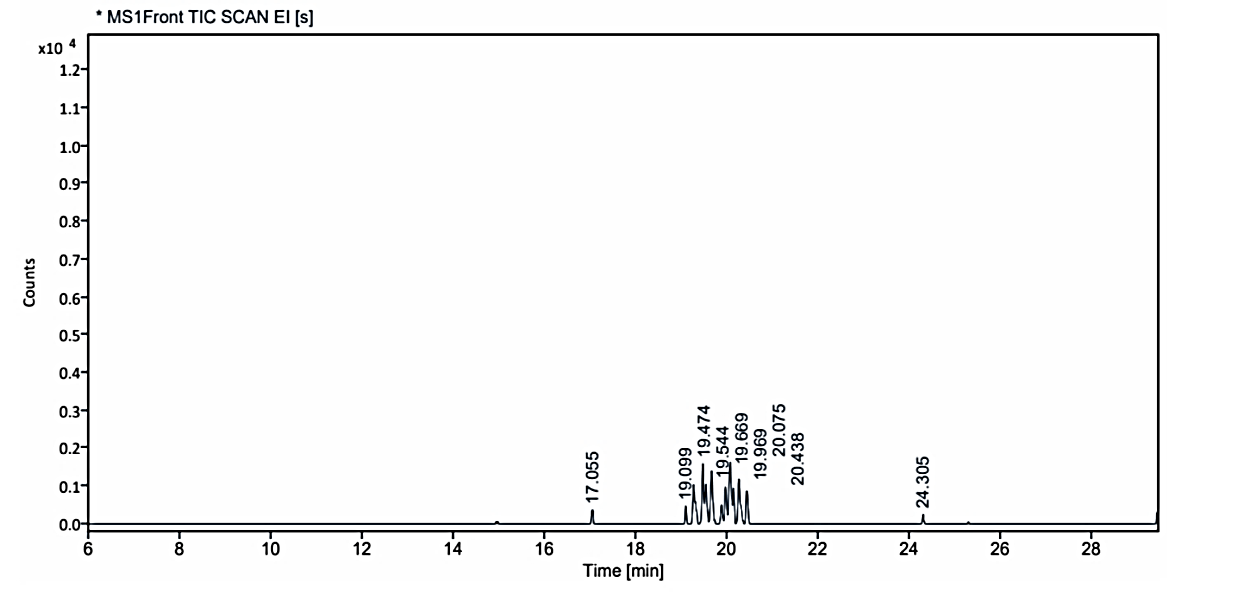
Fig. 1.3: Gas chromatogram showing the isolated compound no. 3 (MO) at Rf value-0.78 on TLC
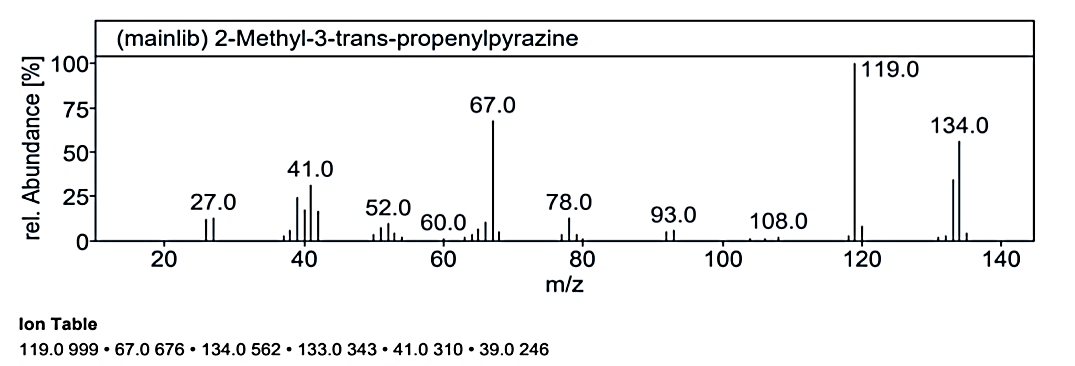
Fig. 1.3a: Mass spectra of the compound no. 3 (MO) at RT value 17.055
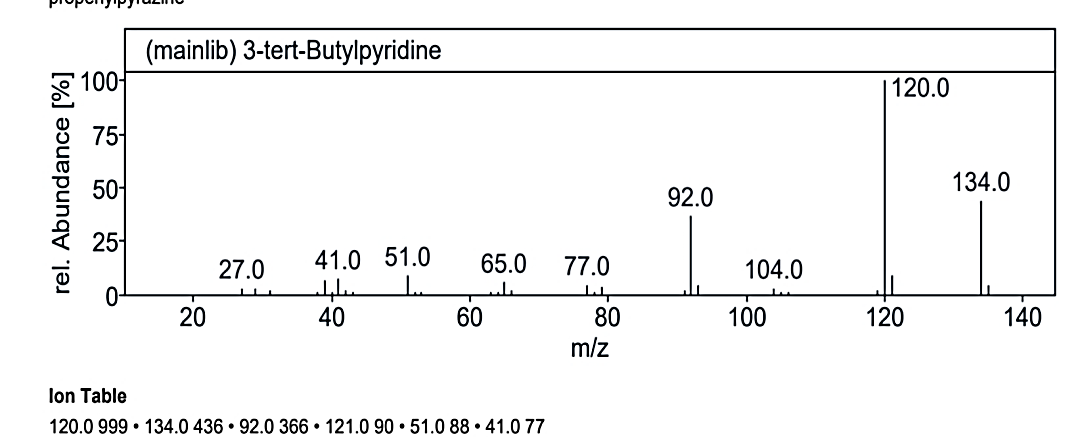
Fig. 1.3b: Mass spectra of the compound no. 3 (MO) at RT value 17.055
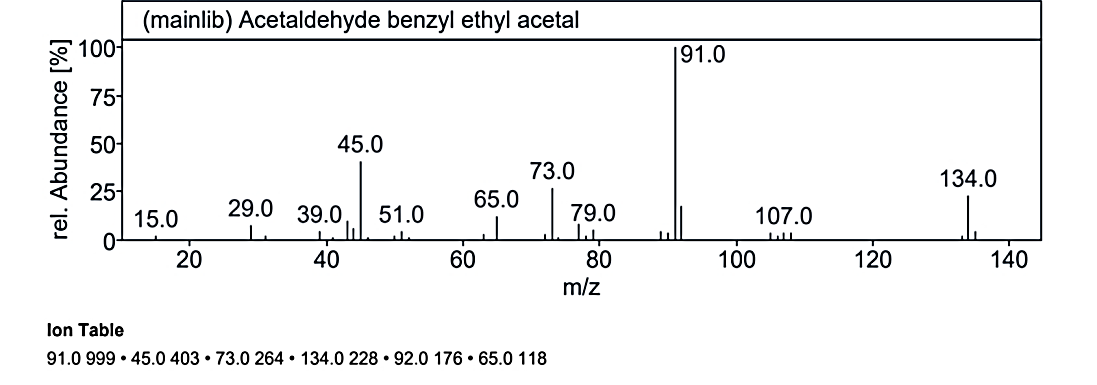
Fig. 1.3c: Mass spectra of the compound no. 3 (MO) at RT value 17.055
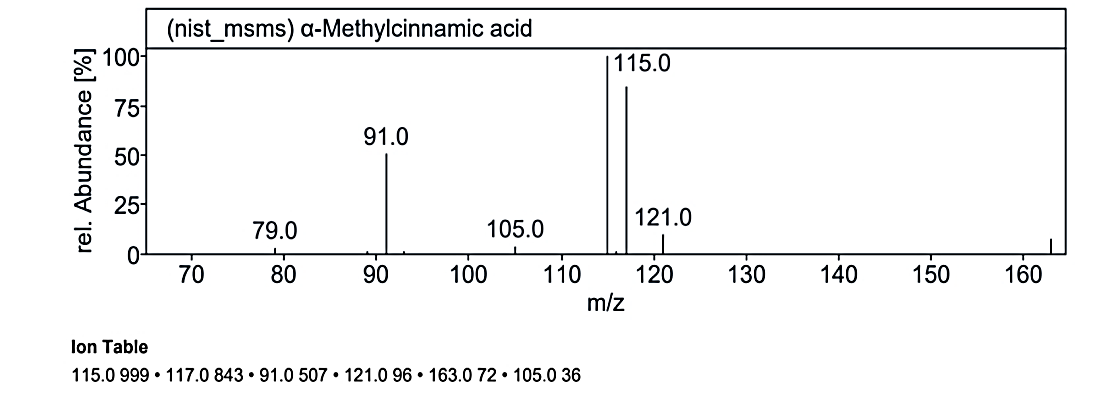
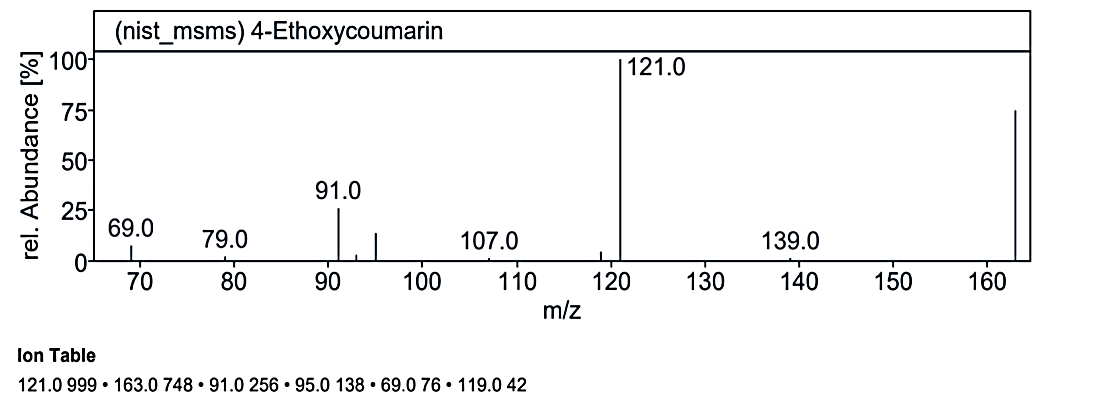
Fig. 1.3d: Mass spectra of the compound no. 3 (MO) at RT value 19.099
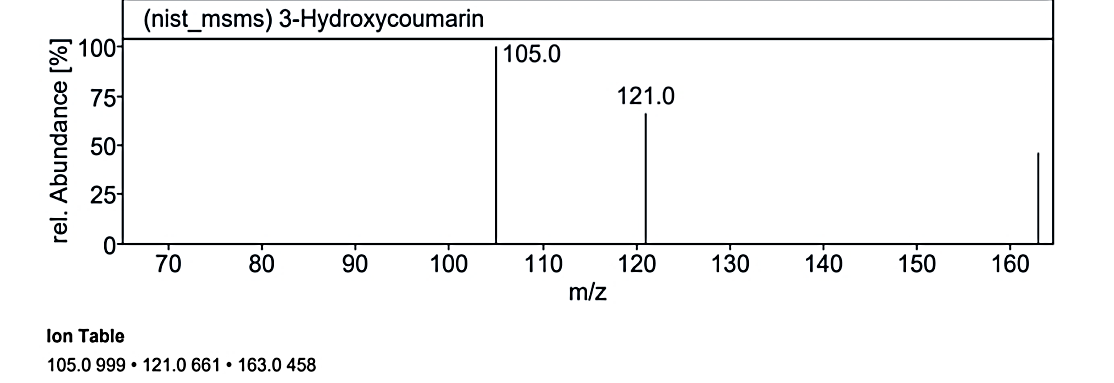
Fig. 1.3e: Mass spectra of the compound no. 3 (MO) at RT value 19.099
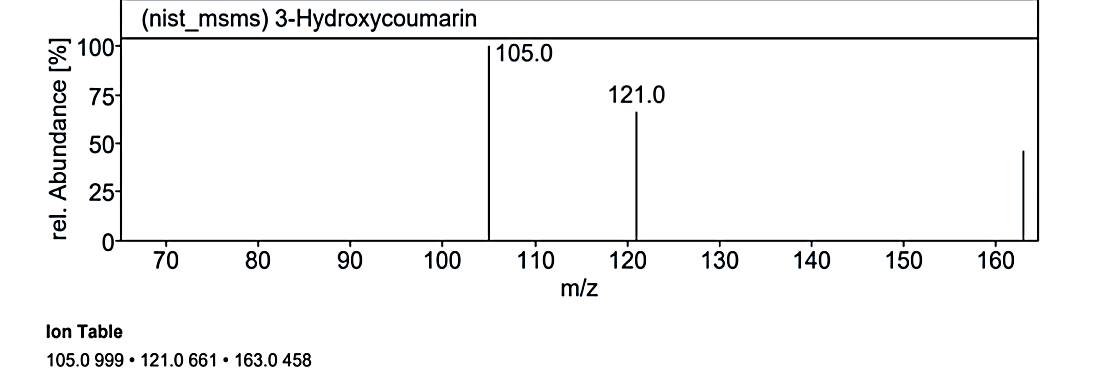
Fig. 1.3f: Mass spectra of the compound no. 3 (MO) at RT value 19.099
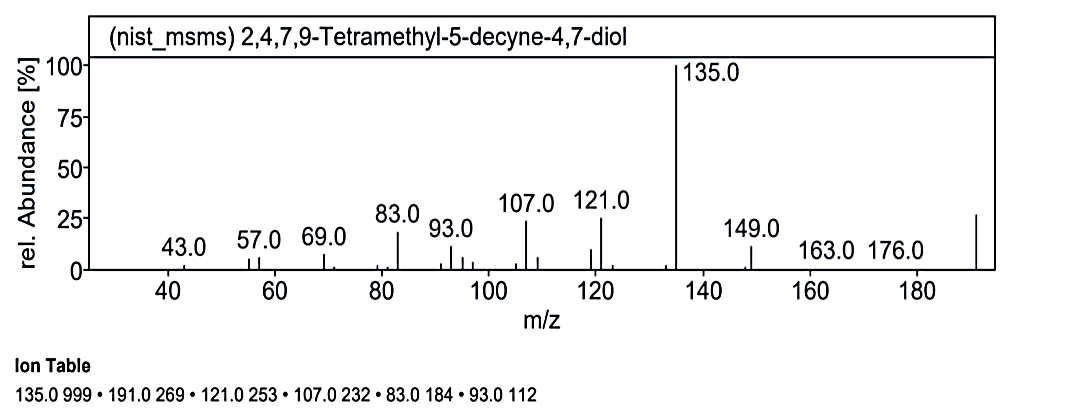
Fig. 1.3g: Mass spectra of the compound no. 3 (MO) at RT value 19.474
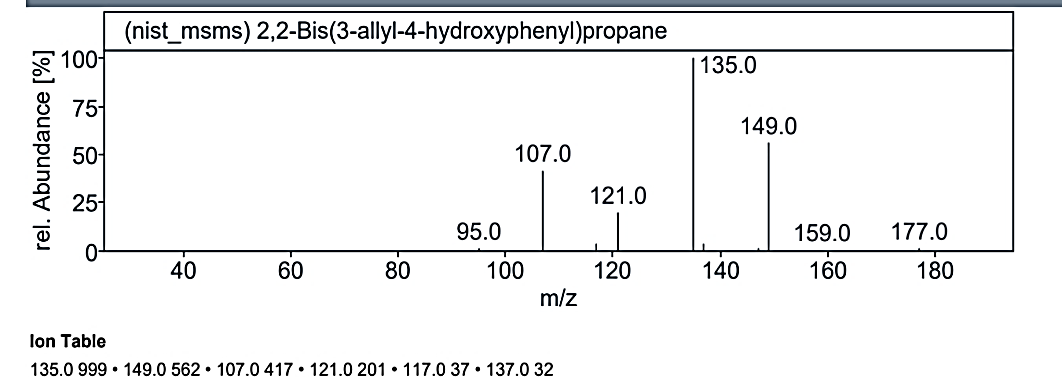
Fig. 1.3h: Mass spectra of the compound no. 3 (MO) at RT value 19.474
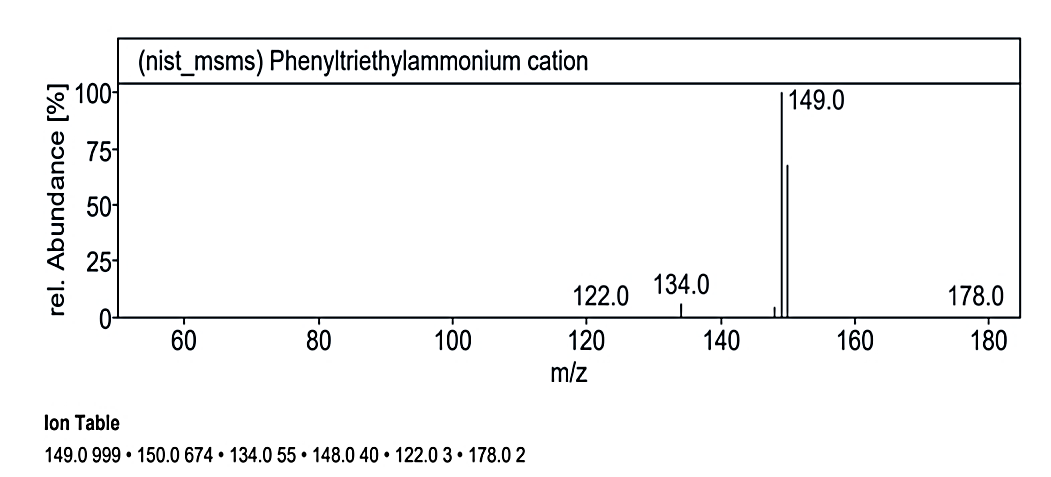
Fig. 1.3i: Mass spectra of the compound no. 3 (MO) at RT value 20.075
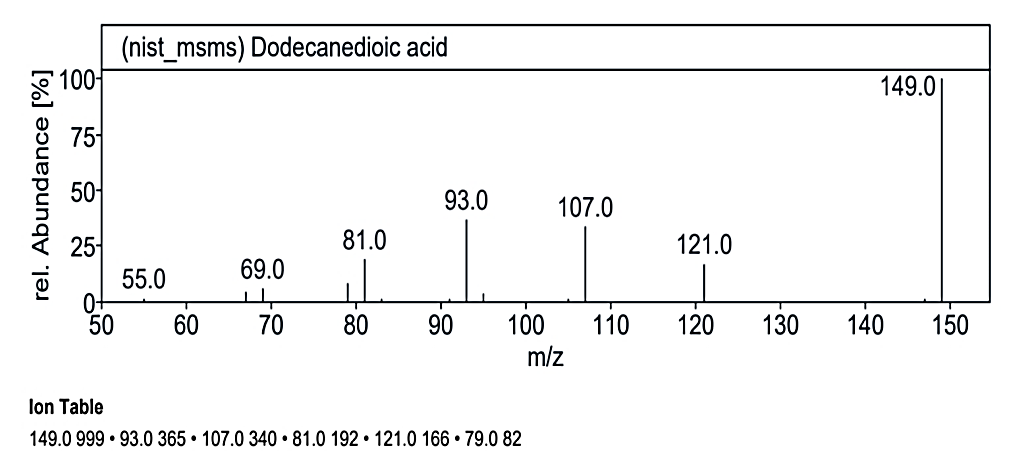
Fig. 1.3j: Mass spectra of the compound no. 3 (MO) at RT value 20.438
GC-MS of the extracts isolated from Coral J. delicata has been performed and the results are presented in fig. 1.1 to 1.3.
The gas chromatograms of the extracts of Coral J. delicata shown in fig. No. 1.1 to 1.3 indicate that there are a large number of peaks. The total run time of the GC was 53.5 min. The temperature of the system was raised up to 350 °C. However, the selected peaks of RT value could be identified and remaining peaks were not identified due to its overlapping or the lack of database in the library as well as any references that are reported till now for the Coral J. delicata extracts. The GC peaks obtained at Rt value of 8.817, 14.954, 17.055, 19.099, 19.474, 20.075, 20.438, and 29.437 were only employed for recording the mass spectra by irradiating the eluents at these Rt values through the electron impact (EI+) source of the Mass spectrometer. Fig. No. 1.1. a to 1.3. j are the mass spectra of eluted compounds at Rt values 8.817, 14.954, 17.055, 19.099, 19.474, 20.075, 20.438, and 29.437 of Coral J. delicata. The M/Z mass peaks obtained for elements at Rt values 8.817, 14.954, 17.055, 19.099, 19.474, 20.075, 20.438, and 29.437 corresponds to the substances of molecular mass of 102.14 g/mol, 132.12 g/mol, 220.25 g/mol, 189.21 g/mol, 222.19 g/mol, 134.09 g/mol, 135.21 g/mol, 180.25 g/mol, 162.19 g/mol, 190.19 g/mol, 162.14 g/mol, 226.35 g/mol, 308.42 g/mol, 178.30 g/mol, 230.30 g/mol.
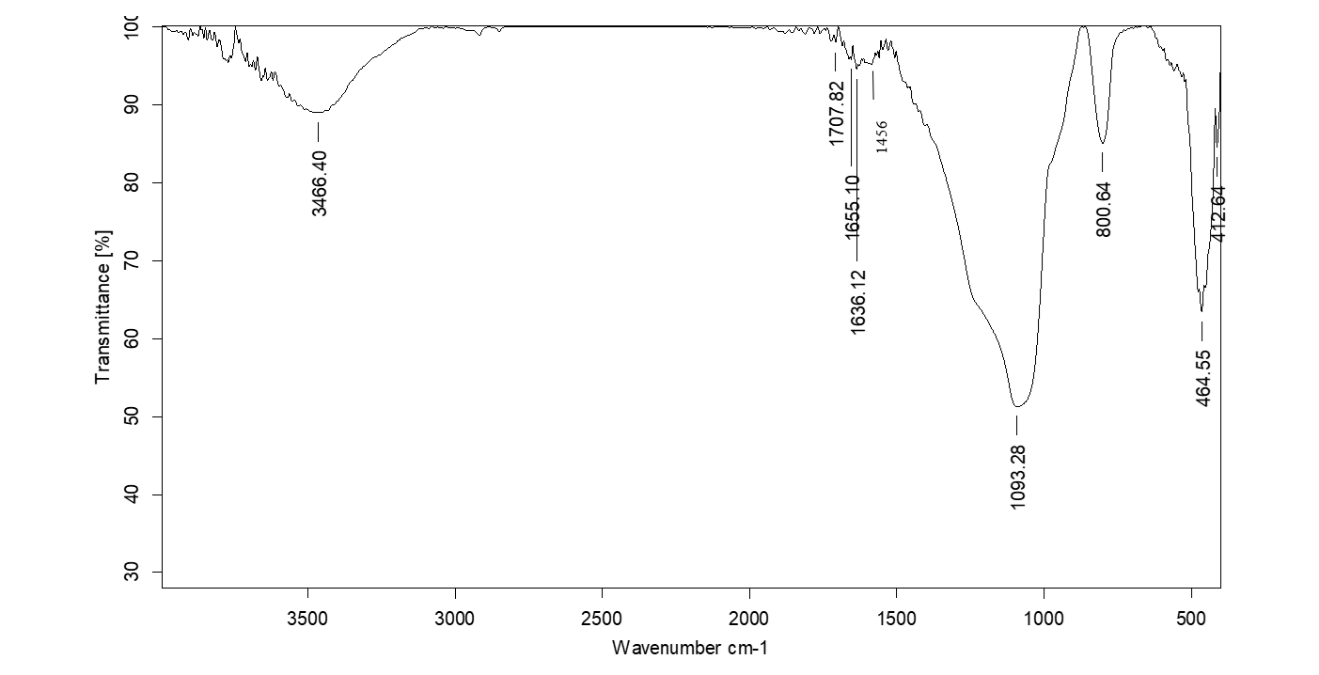
Fig. 2.3: FTIR spectra of the isolated compound No. 3 (MO)-0.78 on TLC
Fragmentation peaks for compound no. 1 (MG)
At an RT value of 29.436 min, the fragmentation peaks were recorded for Compound No. 1 (MG). For the mass spectra with a molecular weight of 102.14 g/mol, the observed peaks included: 102.0 M/Z (10%), 87.0 M/Z (2%), 78.0 M/Z (2%), 74.0 M/Z (80%), 71.0 M/Z (10%), 60.0 M/Z (2%), 55.0 M/Z (5%), and 44.0 M/Z (100%). Similarly, for the mass spectra with a molecular weight of 132.12 g/mol, the peaks were observed at 131.0 M/Z (5%), 113.0 M/Z (2%), 87.0 M/Z (25%), and 74.0 M/Z (100%).
Fragmentation peaks for compound no. 2 (MY)
`The analysis at RT value 8.817 min for Compound No. 2 (MY) yielded peaks for a molecular weight of 220.25 g/mol. The fragmentation pattern showed peaks at 220.0 M/Z (5%), 176.0 M/Z (70%), 175.0 M/Z (100%), 135.0 M/Z (2%), 16.0 M/Z (2%), 105.0 M/Z (2%), and 71.0 M/Z (2%). Another mass spectrum for 189.21 g/mol displayed peaks at 191.0 M/Z (5%), 190.0 M/Z (5%), 176.0 M/Z (10%), 175.0 M/Z (100%), 162.0 M/Z (5%), and 147.0 M/Z (5%). At RT value 14.954 min, the molecular weight of 222.19 g/mol showed peaks at 221.0 M/Z (5%), 206.0 M/Z (22%), 191.0 M/Z (100%), 163.0 M/Z (20%), 150.0 M/Z (5%), 135.0 M/Z (10%), and 107.0 M/Z (2%).
Fragmentation peaks for compound no. 3 (MO)
At RT value 17.055 min, Compound No. 3 (MO) showed fragmentation peaks for a molecular weight of 134.09 g/mol at 134.0 M/Z (50%), 133.0 M/Z (32%), 119.0 M/Z (100%), 108.0 M/Z (2%), 93.0 M/Z (5%), 78.0 M/Z (15%), 67.0 M/Z (65%), 52.0 M/Z (10%), 41.0 M/Z (35%), 39.0 M/Z (24%), and 27.0 M/Z (15%). For the molecular weight of 135.21 g/mol, the peaks included 134.0 M/Z (35%), 121.0 M/Z (7%), 120.0 M/Z (100%), 104.0 M/Z (4%), 92.0 M/Z (30%), 77.0 M/Z (3%), 65.0 M/Z (5%), 51.0 M/Z (10%), and 41.0 M/Z (8%). The molecular weight of 180.25 g/mol displayed peaks at 179.0 M/Z (7%), 134.0 M/Z (25%), 107.0 M/Z (2%), 92.0 M/Z (18%), 91.0 M/Z (100%), 79.0 M/Z (5%), 73.0 M/Z (25%), 65.0 M/Z (15%), 51.0 M/Z (5%), 45.0 M/Z (40%), and 15.0 M/Z (1%).
At RT value 19.099 min, for 162.19 g/mol, peaks included 163.0 M/Z (4%), 121.0 M/Z (10%), 117.0 M/Z (85%), 115.0 M/Z (100%), 105.0 M/Z (5%), and 91.0 M/Z (50%). For 190.19 g/mol, the peaks observed were 191.0 M/Z (16%), 163.0 M/Z (75%), 139.0 M/Z (1%), 121.0 M/Z (100%), 119.0 M/Z (3%), 95.0 M/Z (20%), 91.0 M/Z (25%), and 69.0 M/Z (5%). Another spectrum for 162.14 g/mol displayed 163.0 M/Z (50%), 121.0 M/Z (75%), and 105.0 M/Z (100%).
At RT value 19.474 min, for 226.35 g/mol, the peaks were observed at 227.0 M/Z (33%), 191.0 M/Z (33%), 149.0 M/Z (15%), 135.0 M/Z (100%), 121.0 M/Z (25%), 107.0 M/Z (23%), and 83.0 M/Z (20%). For 308.42 g/mol, the peaks were 309.0 M/Z (26%), 149.0 M/Z (50%), 137.0 M/Z (4%), 135.0 M/Z (100%), 121.0 M/Z (23%), 117.0 M/Z (4%), and 107.0 M/Z (33%).
At RT value 20.075 min, for 178.30 g/mol, the fragmentation peaks were 178.0 M/Z (2%), 150.0 M/Z (62%), 149.0 M/Z (100%), 148.0 M/Z (4%), 134.0 M/Z (5%), and 122.0 M/Z (2%). Lastly, at RT value 20.438 min, for 230.30 g/mol, the peaks observed were 231.0 M/Z (16%), 149.0 M/Z (100%), 121.0 M/Z (18%), 107.0 M/Z (28%), 93.0 M/Z (33%), 81.0 M/Z (20%), and 79.0 M/Z (6%).
FTIR analysis
The FTIR studies are carried out in KBr pallets. Each of the compounds isolated at Rf values at MG-0.38, MY-0.48 and MO-0.78 on TLC for each of the isolated extracts gave the IR spectra’s as shown in fig. 2.1 to 2.3. All the IR spectra of the compounds at the respective Rf values mentioned above are found to be dissimilar.
The wave numbers of some of the important IR peaks along with their intensity, type of vibration and probable groups present in the respective compounds isolated at Rf values MG-0.38, MY-0.48 and MO-0.78 are shown in table 1 to 3.
Table 1: Correlation of IR spectra of the compound isolated from Coral J. delicata at Rf value 0. 38
| Wavenumber (cm⁻¹) | Intensity | Possible functional group | Description |
| 3482 | Broad | O–H or N–H stretching | Hydroxyl group or amine |
| 2926 | Moderate | C–H stretching | Aliphatic C–H (e. g., –CH₃, –CH₂) |
| 1600 | Sharp | C=C or C=N stretching | Aromatic ring or imine group |
| 1380 | Medium | C–N stretching or bending | Amine or imine group |
| 1020 | Medium | C–O or C–N stretching | Ether or amine group |
Table 2: Correlation of IR spectra of the compound isolated from coral J. delicata at Rf value 0. 48
| Wavenumber (cm⁻¹) | Intensity | Possible functional group | Description |
| 3482 | Broad | O–H or N–H stretching | Hydroxyl group or amine |
| 1655 | Sharp | C=O or C=N stretching | Carbonyl group (e. g., ketone, amide) or imine group |
| 1089 | Medium | C–O or C–N stretching | Ether, ester, or amine |
| 802 | Sharp | C–H bending (aromatic) | Aromatic ring presence |
| 464 | Sharp | C–S bending or other fingerprint | - |
Table 3: Correlation of IR spectra of the compound isolated from coral J. delicata at Rf value 0. 78
| Wavenumber (cm⁻¹) | Intensity | Possible functional group | Description |
| 3466 | Broad | O–H or N–H stretching | Hydroxyl group or amine |
| 1707 | Sharp | C=O stretching | Carbonyl group (e. g., carboxylic acid, ester, ketone) |
| 1636 | Medium | C=C or C=N stretching | Aromatic ring or imine group |
| 1456 | Medium | C–H bending (CH₂ or CH₃) | Aliphatic C–H bending |
| 1093 | Medium | C–O or C–N stretching | Ether, ester, or amine group |
| 800 | Sharp | C–H bending (aromatic) | Presence of an aromatic ring |
| 464 | Sharp | C–Cl bending or other fingerprint | Possible halogen (e. g., Cl) presence |
FTIR technique requires very pure samples. Therefore, preparative TLC was carried out to isolate the pure compounds against Coral J. delicata at Rf values at MG-0.38, MY-0.48 and MO-0.78, respectively. The IR spectra of these compounds were recorded and are shown in table 1-3 based on the wave number, intensity of IR peaks and the types of vibration of the IR bands, the probable functional groups present in the compounds were evaluated with the help of standard textbooks [14, 15].
Research conducted by [16] on the gorgonian coral B. violaceum contains a new secondary metabolite briarane diterpenoid, namely briaviolides K–N. The coral P. acerosa contains pseudopteran diterpenes, pseudopterolides [17]. The coral P. elisabethae extract was fractionated with silica gel to produce F-1 pseudopterosins, PsQ, PsS, and PsU, F-2 fraction, amphilectosins A and B, PsG, PsK, PsP, and PsT as well as seco-pseudopterosins seco-PsJ and seco-PsK, and F-3 fraction, elisabethatrienol, 10-acetoxy-9-hydroxy-and 9-acetoxy-10-hydroxy-amphilecta-8,10,12,14-tetraenes and amphilecta-8-11,14-triene-9,10-dione[18]. Coral Heteroxenia fuscescens gorgonian contains secondary metabolites of new sterol types, heterofuscesterols A and B, and 3β, 5α, 6β-trihydroxyandrosta-17-one [19]. The gorgonian coral Rumphella sp. extracted using acetone as a solvent containing new hydroperoxy steroids called xidaosteroids A and B 5α,8αepidioxyergosta-6,9(11)-diene-3β-ol, 5α,8α-epidioxyergosta-6,9(11)-diene-3β-ol,3β-hydroxy5α,6α-epoxy-7-megastigmen-9-one, grasshopper 3 ketone, (3R)-4-[(2R,4S)-4-acetoxy-2-hydroxy-2,6,6-trimethylcyclohexylidene] but-3-en-2-one [20]. The type of gorgonian coral V. corona studied by [21] contains secondary metabolites of-lactone (49),γ-lactone (48), 3β,20R-dihydroxycholest-5,22-dien-24-oic acid γsteroids, namely verrucorosteroid A–F, verrucorosteron, 3β-acetyl-20R-hydroxycholest5,22-dien-24-oic acid suberoretisteroids A–C, (22E)-3β,25-dihydroxycholest-5,22-dien-24-one, 5,6α-epoxy-3βhydroxy-(22E)-ergosta-8,22-dien-7-one, 5,6α-epoxy-3β-hydroxy-(22E)-ergosta-8,22-dien-7-one, (22E,24S)-24-methyl-5α-cholesta-7,22-diene-3β,5,6β,9-tetraol, and (22E,24S)-24-methyl-5αcholesta-7, 22-diene-3β,5,6β-triol. The coral P. americana has extracted using methanol, which produced two types of sterols that have rarely found, namely ameristerenol A and B [22]. Pinnigorgia sp. obtain 11-acetoxy-9, 11-secosterol, pinnisterol D–J, sterol, and pinnisterol A [23]. Subergorgia suberosa coral, based on research by Cheng et al. (2016) has reported containing five new pregnan steroid types called subergol T–X and three others known as analogs. The coral S. rubra gorgonian has reported containing a new-3-ketosteroid characterized by 9-OH, subergosterone A–C together with five steroids, namely 9αhydroxycholest-1-en-3-one, pregna-1, 4, 20-trien-3-one, cholesta-1,4-dien-3-one, dendronesterone C, and ergosta-1,4-dien-3-one [24]. The coral Eunicella singularis has been studied to contain sterols, namely cholest-5-ene-3β,7α-diol; (22E)-cholesta-5, 22-diene-3β,7α-diol; ergosta-5,24 diene-3β,7α-diol; and cholesta-5, 22-diene-3βol [25]. Echinomuricea sp. gorgonian contains a new sterol, namely 6-epi-yonarasterol B, which has extracted using methanol–dichloromethane [26]. The coral D. griffini was extracted with ethanol; results contained two new sterol compounds, namely griffiniteron and griffinipregnon. Research conducted by [27] on gorgonian coral Paramuricea clavata and reported that it contains two new alkaloids, namely 2-bromo-N-methyltryptamine, 3-bromo-N-methyltyramine [28]. Terpenoid briarane Briaviolide K–N contained in gorgonian B. violaceum was helpful as a pro-inflammatory inhibitor in iNOS targets with values LPS cells. Briaviolide l effectively reduced iNOS and COX-2 with values. However, it is different from briaviolide K, which is inactive in reducing the two pro-inflammatory enzymes' expression, even though the hydroxy group is very crucial because it plays an important role in providing biological activity [29]. Excavatolide B metabolite was isolated from B. excavatum gorgonian coral, which has significant activity in inhibiting iNOS protein expression [30]. Echinolabdane A and 6-epi-yonarasterol B. Echinolabdane A secondary metabolite, have found in Echinomuricea sp. which can inhibit superoxide anion derivatives. The secondary metabolite 6-epi-yonarasterol B exhibits a significant inhibitory effect on the superoxide anion derivative and elastase release by human neutrophils [31]. Caryophyllene sesquiterpenoids the gorgonian coral of R. antipathies showed that the caryophyllene derivatives of the sesquiterpenoid type, rumphellol A and B, had anti-inflammatory effects when tested in vitro [32]. Pseudopteran diterpene gorgonian coral showed Pseudopterolide can inhibit the production of inflammatory mediators NO, TNF-α, IL-6, IL-1β, and IP-10 induced by LPS a methoxy functional group at C-9 [33]. Diterpenoid type eunicellin Secondary metabolite palmonine F sourced from gorgonian E. singularis has anti-inflammatory activity when tested using the carrageenan-induced rat paw edema model and acetic acid writhing test mice [34]. Secondary metabolites H. fuscescens have cytotoxic activity [3-(4, 5-dimethylazol-2-il)-2,5-diphenyltetrazolium bromide] with cancer cell lines MCF-7 (human breast adenocarcinoma) and OVK-18 (endometrioid ovarian carcinoma). Heterofusceterpene A shows cytotoxic activity on MCF-7 and OVK-18 [35]. The study carried out by [36] isolated bioactive compounds from two sponges Dysidea herbacea, Sigmadocia pumila by using GC-MS techniques. They found different 11 secondary metabolites which are widely used in cosmetics, pharmaceuticals and other industries [37]. Conducted the experiment on the anti-inflammatory effect of lipid extract of sea pen Virgularia gustavianain mice showed strong anti-inflammatory effects even at low dose which is due to the presence of arachidonic acid in the compounds [38]. Reviewed on therapeutic and pharmaceutical effects of coelenterate toxins and they found that the coelenterate toxins have different biological activities such as cytolytic or neurotoxic, hemolytic, anti-parasitic activity, α-amylase inhibitor activity, and analgesic activity, anti-cancerous and antitumor activity, anti-inflammatory and antimicrobial activity. They further suggested that the coelenterate toxins could be used to develop potential therapeutic drugs for various human diseases and disorders. [39] Worked on bioactive compounds from sponge Suberites carnosus (Johnston) collected from West coast of Mumbai, India. They isolated ten compounds by using GC-MS and FTIR techniques, these compounds are 6-Fluoro 2-trifluromethylbenzoic acid,2,3-dichlorophenyl ester, Eicosane 3-cyclohexyl, Phosphine imide, P,P,P,-tris (p-chlorophenyl)-Nphenyl-, Dimethylhyl hexavinyl octasilsesquioxane, Hexanoic acid, hexadecyl ester, Hexadecanoic acid, 2-hydroxy1-(hydroxymethyl)ethyl ester, 9, 19-Cyclolanostan-3-ol, acetate,(3β), Tetracosane, 3-ethyl-, 11, 14-Eicosadienoic acid, methyl ester, Triacontane,11,20-didecyl respectively. They further concluded that these compounds showed biomedical properties such as skin irritant, fatty, metabolite, masking and perfuming agents, highly corrosive and chemotaxonomic significance.
The distinct and well-separated three compounds were isolated from crude extracts of coral J. delicata. The preparative HPTLC was performed to isolate pure compounds from the extracts. Analysis of the extracts was performed to find out the nature of compounds by GC-MS and FTIR technique. The results conclude that the compounds isolated at Rf values at MG-0.38, MY-0.48 and MO-0.78 corresponds to the molecular weights of compounds identified as-(Ethyl aminomethyl formimidate); (Gly-Gly); (2-(2-Pyridyl)-4 methylthiazole-5-carboxylic acid); (7-Methoxy-2-methylquinolin-4-ol); (Fraxidin); (2-methyl-3-trans-propenylpyrazine); (3-tert-Butylpyridine); (Acetaldehyde benzyl ethyl acetal); (α-Methylcinnamic acid); (4-Ethoxycoumarin); (3-Hydroxycoumarin); (2, 4, 7, 9-Tetramethyl-5-decyne-4, 7-diol); (2,2-Bis(3-allyl-4-hydroxyphenyl) propane); (Phenyltriethylammonium cation); (Dodecanedioic acid).
Table 4: Showing names of the compounds, their molecular weights, molecular formula, and structures of the compounds isolated from coral J. delicata
| S. No. | Name of the compound | Molecular weight | Molecular formula | Structure |
| 1. | Ethyl aminomethylformimidate | 102.14 g/mol | C4H10N2O | 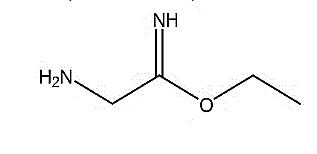 |
| 2. | Gly-Gly | 132.12 g/mol | C4H8N2O3 | 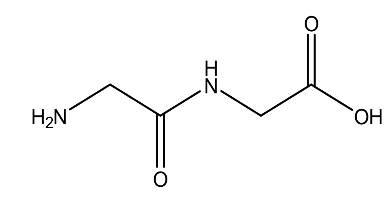 |
| 3. | 2-(2-Pyridyl)-4 methylthiazole-5-carboxylic acid | 220.25 g/mol | C10H8N2O2S | 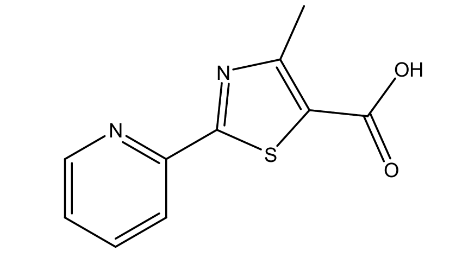 |
| 4. | 7-Methoxy-2-methylquinolin-4-ol | 189.21 g/mol | C11H11NO2 | 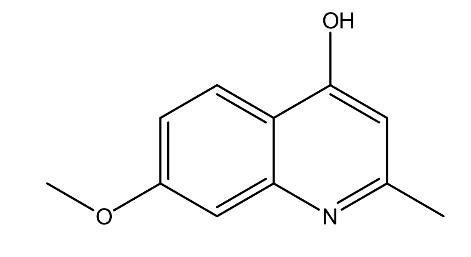 |
| 5. | Fraxidin | 222.19 g/mol | C11H10O5 | 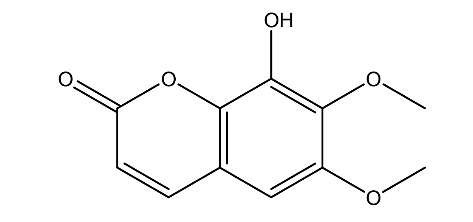 |
| 6. | 2-Methyl-3-trans-propenyl pyrazine | 134.09 g/mol | C8H10N2 | 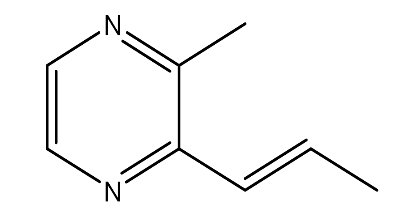 |
| 7. | 3-tert-Butylpyridine | 135.21 g/mol | C9H13N | 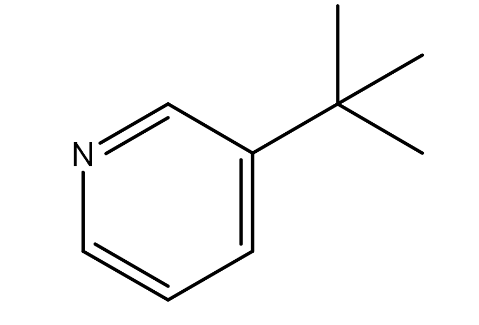 |
| 8. | Acetaldehyde benzyl ethyl acetal | 180.25 g/mol | C11H16O2 | 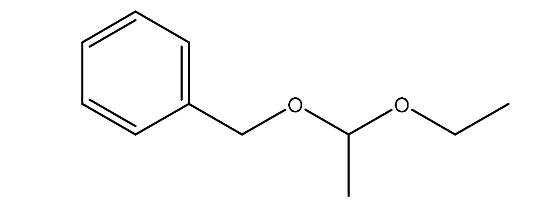 |
| 9. | α-Methylcinnamic acid | 162.19 g/mol | C10H10O2 | 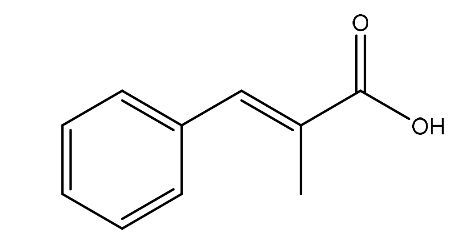 |
| 10. | 4-Ethoxycoumarin | 190.19 g/mol | C11H10O3 | 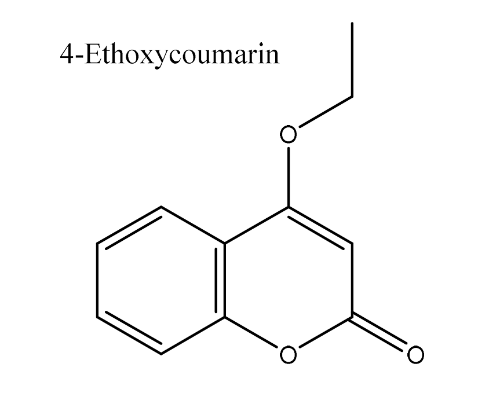 |
| 11. | 3-Hydroxycoumarin | 162.14 g/mol | C9H6O3 | 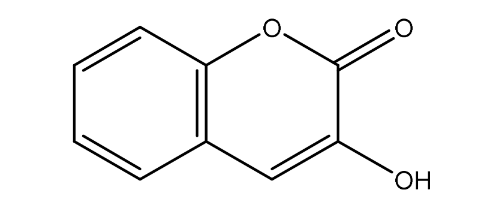 |
| 12. | 2,4,7,9-Tetramethyl-5-decyne-4,7-diol | 226.35 g/mol | C14H26O2 | 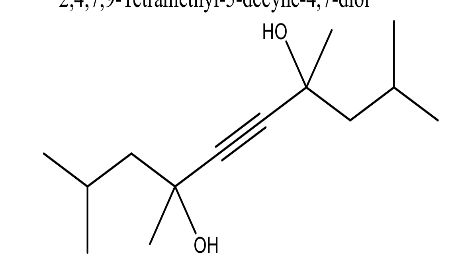 |
| 13. | 2,2-Bis(3-allyl-4-hydroxyphenyl) propane | 308.42 g/mol | C21H24O2 | 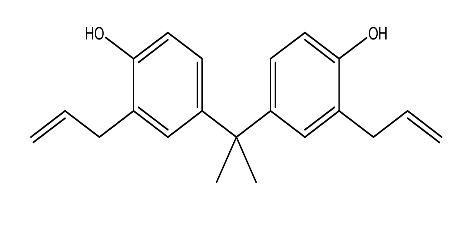 |
| 14. | Phenyltriethylammonium cation | 178.30 g mol | C12H20N+ | 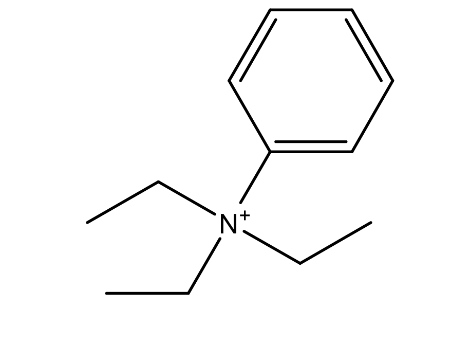 |
| 15 | Dodecanedioic acid | 230.30 g/mol | C12H22O4 | 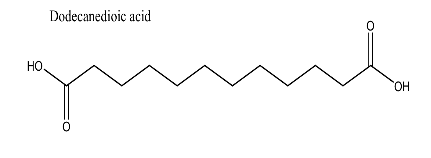 |
CONCLUSION
From the above results, it is concluded that the GC-MS and FTIR analysis of J. delicata extracts has revealed a comprehensive chemical profile, highlighting distinct fragmentation patterns, functional groups, and promising biological activities. These findings emphasize the potential of this coral as a valuable source of bioactive compounds for pharmaceutical and therapeutic applications. Further studies, including detailed structural elucidation, bioassays, and molecular-level research, are necessary to confirm their mechanisms of action and clinical relevance. Additionally, safety and efficacy assessments are crucial to support the development of new pharmaceutical products aimed at improving human health.
ACKNOWLEDGEMENT
The authors thank Dr. Swapnaja Mohite, Professor and Head, Department of Fisheries Biology, College of Fisheries, Shirgaon, Ratnagiri, for final species identification and confirmation. The thanks are also due to The Director, Principal Chief Conservator of Forest, Nagpur and The Director, Maharashtra State Biodiversity Board, Nagpur, for permitting the collection of species. Thanks are also due to the Director, Anchrom Test Lab, Mulund, Mumbai, for providing HPTLC (High Performance Thin Layer Chromatography) facilities. Special thanks to the Director, SAIF, IIT Madras, for GC-MS analysis, and the Director, SAIF, IIT Bombay, for providing FTIR facilities to support the analytical studies.
FUNDING
Authors are thankful to Mahatma Jyotiba Phule Research and Training Institute (MJPRF), Nagpur, Govt. of Maharashtra, for their financial support and cooperation for the research.
ETHICAL STATEMENT
Principal Chief Conservator of Forest, Nagpur (Desk-22(8)/Res/CR-25(22-23)/1431/(22-23) and final approval was taken from the Maharashtra State Biodiversity Board, Nagpur (MSBB/Desk-5/825/2022-23) for collection of J. delicata samples.
AUTHORS CONTRIBUTIONS
Meenakshi Prakash Borate: data collection, analysis and writing. Prof. Dr. G. V. Zodape: Conceptualization and supervision.
CONFLICT OF INTERESTS
Authors have no conflict of interest
REFERENCES
Zhang W, Guo YW, Gu Y. Secondary metabolites from the South China Sea invertebrates: chemistry and biological activity. Curr Med Chem. 2006;13(17):2041-90. doi: 10.2174/092986706777584960, PMID 16842196.
Yang F, Li SW, Zhang J, Liang LF, Lu YH, Guo YW. Uncommon nornardosinane seconeolemnane and related sesquiterpenoids from Xisha soft coral Litophyton nigrum. Bioorg Chem. 2020 Mar;96:103636. doi: 10.1016/j.bioorg.2020.103636, PMID 32045775.
Aratake S, Tomura T, Saitoh S, Yokokura R, Kawanishi Y, Shinjo R. Soft coral Sarcophyton (Cnidaria: Anthozoa: Octocorallia) species diversity and chemotypes. PLOS One. 2012;7(1):e30410. doi: 10.1371/journal.pone.0030410, PMID 22272344.
Fabricius KE, Klumpp DW. Widespread mixotrophy in reef-inhabiting soft corals: the influence of depth, and colony expansion and contraction on photosynthesis. Mar Ecol Prog Ser. 1995;125(1-3):195-204. doi: 10.3354/meps125195.
Van Alstyne KL, Wylie CR, Paul VJ, Meyer K. Antipredator defenses in tropical pacific soft corals (Coelenterata: Alcyonacea). I. Sclerites as defenses against generalist carnivorous fishes. Biol Bull. 1992;182(2):231-40. doi: 10.2307/1542116, PMID 29303665.
Dobretsov S, Al Wahaibi AS, Lai D, Al Sabahi J, Claereboudt M, Proksch P. Inhibition of bacterial fouling by soft coral natural products. Int Biodeterior Biodegrad. 2015 Mar;98:53-8. doi: 10.1016/j.ibiod.2014.10.019.
Sammarco PW, La Barre S, Coll JC. Defensive strategies of soft corals (Coelenterata: Octocorallia) of the Great Barrier Reef: III. The relationship between ichthyotoxicity and morphology. Oecologia. 1987;74(1):93-101. doi: 10.1007/BF00377351, PMID 28310420.
Ellithey MS, Lall N, Hussein AA, Meyer D. Cytotoxic and HIV-1 enzyme inhibitory activities of red sea marine organisms. BMC Complement Altern Med. 2014;14(1):77. doi: 10.1186/1472-6882-14-77, PMID 24568567.
Kelman D, Kushmaro A, Loya Y, Kashman Y, Benayahu Y. Antimicrobial activity of a red sea soft coral Parerythropodium fulvum fulvum: reproductive and developmental considerations. Mar Ecol Prog Ser. 1998 Aug;169:87-95. doi: 10.3354/meps169087.
Harder T, Lau SC, Dobretsov S, Fang TK, Qian PY. A distinctive epibiotic bacterial community on the soft coral Dendronephthya sp. and antibacterial activity of coral tissue extracts suggest a chemical mechanism against bacterial epibiosis. FEMS Microbiol Ecol. 2003;43(3):337-47. doi: 10.1111/j.1574-6941.2003.tb01074.x, PMID 19719665.
Gomaa MN, Soliman K, Ayesh A, Abd El Wahed A, Hamza Z, Mansour HM. Antibacterial effect of the Red Sea soft coral Sarcophyton trocheliophorum. Nat Prod Res. 2016;30(6):729-34. doi: 10.1080/14786419.2015.1040991, PMID 26186031.
Zhao M, Yin J, Jiang W, Ma M, Lei X, Xiang Z. Cytotoxic and antibacterial cembranoids from a South China Sea soft coral, Lobophytum sp. Mar Drugs. 2013;11(4):1162-72. doi: 10.3390/md11041162, PMID 23552878.
Pereira I, Severino P, Santos AC, Silva AM, Souto EB. Linalool bioactive properties and potential applicability in drug delivery systems. Colloids Surf B Biointerfaces. 2018 Nov 1;171:566-78. doi: 10.1016/j.colsurfb.2018.08.001, PMID 30098535.
Xu JH, Lai KH, Su YD, Chang YC, Peng BR, Backlund A. Briaviolides KN, new briarane-type diterpenoids from cultured octocoral Briareum violaceum. Mar Drugs. 2018;16(3):75. doi: 10.3390/md16030075, PMID 29495481.
Dyer. Applications of adsorption spectroscopy of organic compounds. Englewood Cliffs: Prentice Hall of India Private Limited; 1974.
Brian F, Antoni J, Hannaford P, Smith WG, Tatchell AR. Textbook of Organic Chemistry. Longman Group, UK Ltd; 1989.
Gonzalez Y, Doens D, Santamaria R, Ramos M, Restrepo CM, Barros De Arruda LB. A pseudopterane diterpene isolated from the octocoral Pseudopterogorgia acerosa inhibits the inflammatory response mediated by TLR-ligands and TNF-alpha in macrophages. PLOS One. 2013;8(12):e84107. doi: 10.1371/journal.pone.0084107, PMID 24358331.
Correa H, Valenzuela AL, Ospina LF, Duque C. Anti-inflammatory effects of the gorgonian Pseudopterogorgia elisabethae collected at the Islands of providencia and San Andres (SW Caribbean). J Inflamm (Lond). 2009;6:5. doi: 10.1186/1476-9255-6-5, PMID 19284567.
Abdelkarem FM, Desoky EK, Nafady AM, Allam AE, Mahdy A, Ashour A. Two new polyhydroxylated steroids from Egyptian soft coral Heteroxenia fuscescens (Fam Xeniidae). Nat Prod Res. 2021;35(2):236-43. doi: 10.1080/14786419.2019.1624958, PMID 31170807.
Yin FZ, Yang M, Li SW, Wu MJ, Huan XJ, Miu ZH. Two new hydroperoxy steroids from the South China Sea gorgonian Rumphella sp. Steroids. 2020 Mar;155:108558. doi: 10.1016/j.steroids.2019.108558, PMID 31866544.
Nam NH, Ngoc NT, Hanh TT, Cuong NX, Thanh NV, Thao DT. Cytotoxic steroids from the Vietnamese gorgonian Verrucella corona. Steroids. 2018;138:57-63. doi: 10.1016/j.steroids.2018.06.006, PMID 30018002.
He YQ, Lee Caplan SL, Scesa P, West LM. Cyclized 9,11-secosterol enol-ethers from the gorgonian Pseudopterogorgia americana. Steroids. 2017 Sep;125:47-53. doi: 10.1016/j.steroids.2017.06.008, PMID 28648587.
Chang YC, Hwang TL, Kuo LM, Sung PJ. Pinnisterols D–J, new 11-acetoxy-9,11-secosterols with a 1,4-quinone moiety from formosan gorgonian coral Pinnigorgia sp. (Gorgoniidae). Mar Drugs. 2017;15(1):11. doi: 10.3390/md15010011, PMID 28067822.
Cao F, Shao C, Wang M, Zhang X, Wang C. Steroids. Steroids. 2015;12:1068.
Deghrigue M, Festa C, Ghribi L, D Auria MV, De Marino S, Ben Jannet H. Anti-inflammatory and analgesic activities with gastroprotective effect of semi-purified fractions and isolation of pure compounds from Mediterranean gorgonian Eunicella singularis. Asian Pac J Trop Med. 2015;8(8):606-11. doi: 10.1016/j.apjtm.2015.07.019, PMID 26321512.
Chung HM, Hong PH, Su JH, Hwang TL, Lu MC, Fang LS. Bioactive compounds from a gorgonian coral Echinomuricea sp. (Plexauridae). Mar Drugs. 2012;10(5):1169-79. doi: 10.3390/md10051169, PMID 22822364.
Penez N, Culioli G, Perez T, Briand JF, Thomas OP, Blache Y. Antifouling properties of simple indole and purine alkaloids from the Mediterranean gorgonian Paramuricea clavata. J Nat Prod. 2011;74(10):2304-8. doi: 10.1021/np200537v, PMID 21939218.
Penez N, Culioli G, Perez T, Briand JF, Thomas OP, Blache Y. Antifouling properties of simple indole and purine alkaloids from the Mediterranean gorgonian Paramuricea clavata. J Nat Prod. 2011;74(10):2304-8. doi: 10.1021/np200537v, PMID 21939218.
Siva SD, Vijayakumar N, Jayaprakash R, Reddi NM. Review on isolation, identification and applications of Simarouba glauca plant. Rasayan J Chem. 2020;13(3):1580-8. doi: 10.31788/RJC.2020.1335793.
Lin YY, Lin SC, Feng CW, Chen PC, Su YD, Li CM. Anti-inflammatory and analgesic effects of the marine-derived compound excavatolide B isolated from the culture type formosan gorgonian Briareum excavatum. Mar Drugs. 2015;13(5):2559-79. doi: 10.3390/md13052559, PMID 25923315.
Chung HM, Hong PH, Su JH, Hwang TL, Lu MC, Fang LS. Bioactive compounds from a gorgonian coral Echinomuricea sp. (Plexauridae). Mar Drugs. 2012;10(5):1169-79. doi: 10.3390/md10051169, PMID 22822364.
Chung HM, Wang WH, Hwang TL, Chen JJ, Fang LS, Wen ZH. Rumphellols A and B, new caryophyllene sesquiterpenoids from a formosan gorgonian coral, Rumphella antipathies. Int J Mol Sci. 2014;15(9):15679-88. doi: 10.3390/ijms150915679, PMID 25192289.
Gonzalez Y, Doens D, Santamaria R, Ramos M, Restrepo CM, Barros De Arruda LB. A pseudopterane diterpene isolated from the octocoral Pseudopterogorgia acerosa inhibits the inflammatory response mediated by TLR-ligands and TNF-alpha in macrophages. PLOS One. 2013;8(12):e84107. doi: 10.1371/journal.pone.0084107, PMID 24358331.
Deghrigue M, Festa C, Ghribi L, D’Auria MV, De Marino S, Ben Jannet H. Anti-inflammatory and analgesic activities with gastroprotective effect of semi-purified fractions and isolation of pure compounds from Mediterranean gorgonian Eunicella singularis. Asian Pac J Trop Med. 2015;8(8):606-11. doi: 10.1016/j.apjtm.2015.07.019, PMID 26321512.
Abdelkarem FM, Desoky EK, Nafady AM, Allam AE, Mahdy A, Ashour A. Two new polyhydroxylated steroids from Egyptian soft coral Heteroxenia fuscescens (Fam.; Xeniidae). Nat Prod Res. 2021;35(2):236-43. doi: 10.1080/14786419.2019.1624958, PMID 31170807.
Thambidurai Y, Habeeb S, Kizhakudan J, DS. Screening of bioactive compounds from unknown marine sponges collected from kovalam, Chennai. Asian J Pharm Clin Res. 2017;10(5):231-6. doi: 10.22159/ajpcr.2017.v10i5.17347.
Sharifi S, Safaeian S. Anti-inflammatory effect of lipid extract of sea pen (Virgularia gustaviana) in mice. Asian J Pharm Clin Res. 2015;8(2):332-4.
Sharma S, Upadhyay RK. Coelenterate toxins its pharmaceutical and therapeutic effects. Int J Curr Pharm Sci. 2021;13(6):11-9. doi: 10.22159/ijcpr.2021v13i6.1912.
Bhadekar NS, Zodape GV. Original article bioactive compounds from sponge Suberites carnosus (Johnston) collected from West Coast of Mumbai, India. Int J Pharm Pharm Sci. 2021;13(6):41-51. doi: 10.22159/ijpps.2021v13i6.40794.