
Int J Pharm Pharm Sci, Vol 17, Issue 9, 15-20Review Article
A CRITIQUE ONMECHANISM OF NIGELLA SATIVA AS AN ANTI-DIABETIC DRUG: FOCUS ON THE THERAPEUTIC DOSE BASED ON ASSORTED EXPLICATIONS
SANA BUTOOL1, SAILAJA RAO P.2*, RAVI KUMAR V.3, SREEDEVI B.4
1,2,4Department of Pharmacology, Teegala Ram Reddy College of Pharmacy, Hyderabad, Telangana, India. 3Department of Pharmacology, MNR College of Pharmacy, Hyderabad, Telangana, India
*Corresponding author: Sailaja Rao P.; *Email: sailajarao476@gmail.com
Received: 10 May 2025, Revised and Accepted: 04 Jun 2025
ABSTRACT
Globally, Diabetic mellitus is a rapidly progressing metabolic disorder and is becoming a worldwide concern with several complications and deaths every year. Despite conventional anti-diabetic drugs, numerous kinds of research are going on to get the best cost-effective therapeutic agents with the least adverse effects for the management of diabetes and its complications. Nigella sativa is a spice with multi-effects on various disorders like anti-diabetic, anti-cancer, immune modulator, anti-microbial, anti-inflammatory, anti-spasmodic, relives pain, bronchodilator, hepato and renal protective, gastro-protective, anti-oxidant properties. Amongst all effects, the anti-diabetic properties remained a cornerstone and was explored. Anti-diabetic effect of N. sativa was due to the presence of Thymoquinone, a major constituent responsible for its effect. Since long ago, studies revealed that the active constituent thymoquinone had a significant reduction in fasting and post-prandial blood glucose levels (glycemic control), probably affecting the pancreatic β-cells, on insulin production and secretion; moreover, lipid profile was shown to be improved in both clinical and preclinical trials. However, there are not many studies on the exact dose to be administered for the therapeutic effect clinically. This review investigated and emphasized the molecular mechanism of N. sativa based on the pre-clinical, clinical and toxicological evaluations. This aimed for the estimation of effective dose of N. sativa therapeutically for healthier out-turn.
Keywords: Nigella sativa, Diabetes mellitus, Mechanism of action, Dose, Side effects
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijpps.2025v17i9.54971 Journal homepage: https://innovareacademics.in/journals/index.php/ijpps
INTRODUCTION
Diabetes mellitus is a chronic endocrine disorder characterized by persistent increase in blood glucose levels (hyperglycemia), polyuria (excess urination), insulin resistance, and pancreatic dysfunction [1]. Diabetes mellitus is associated with a plethora of complications of various organs, majorly, kidneys, eyes, and heart and nervous. There is a high risk of heart disease and stroke, around 50% of diabetic individuals die from cardiovascular diseases [2]. The mortality rate is highly noticed in developing countries with lower and middle income [3]. India is at its peak and now become a diabetic capital globally. It is estimated that the number of people affected with diabetes has reached 300 million by this year and around 700 million people will be affected by 2045 [4]. As a consequence of this analyzed epidemiological data, the International Diabetes Federation (IDF, 2021) continued studies on pathogenesis and treatment for diabetes mellitus [5, 6]. In countries like India, all are looking for affordable medications to manage diabetes with the fewest side effects. In the current review, exploration has been made on herbal/unani drugs, which have long been in the subject of research for their effects on various chronic illnesses.
Generally, as diabetes is considered as chronic lifestyle disorder which exists for a whole life time, there can be every chance of incessant high blood glucose levels that subsequently can generate free radicals and further lead to the genesis of oxidative stress in different parts of the body [7]. This will additionally may create many complications on different organs of the body like heart, brain, kidneys, eyes and blood vessels. Hence, there is a mandatory requirement for such a drug which targets these free radicals and have an action at molecular level is always preferred as they combat the root cause of a disease and have sufficient therapeutic effect.
Since ancient times, medicinal plants have been used by mankind as traditional treatments for a wide range of acute and chronic ailments. According to the World Health Organization (WHO), more than three-fourths of the population in resource-constrained nations rely on medicinal plants. This might be because of the inaccessibility and cost of allopathic medicines [3, 9].
Amongst the plethora of medicinal plants, Nigella sativa (NS) is considered an excellent ancient herb with miraculous therapeutic effects, which belongs to the family Ranunculaceae. It is commonly called as black cumin (or) kalonji [10, 11], and has been used in the ancient system of medicine for its potential benefits [12]. These therapeutic effects of N. sativa are due to the presence of the compound Thymoquinone. Thymoquinone (2-Isopropl-5-methyl benzo-1,4-quinone) is an active ingredient present in the seeds of Nigella sativa [13].
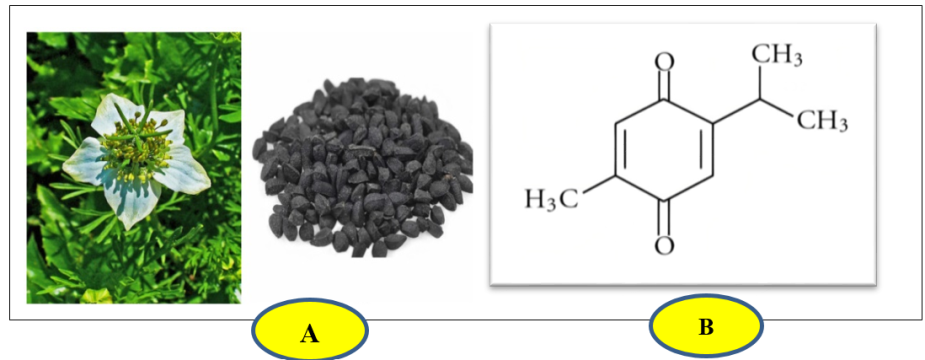
Fig. 1: A) Nigella sativa, flower and seeds [14], B) The chemical structure of Thymoquinone [14]
Nigella sativa is considered a Prophetic medicine in Islam and has a wide variety of therapeutic uses in various illnesses because of which it is known as ‘Habbah Sawda’ or ‘Habbat el Baraka’ (in Arabic, which means ‘Seeds of blessing’), ‘Panacea’ (in Latin, translated as ‘cure-all’), ‘kalo jeera’ (in Bangladesh), ‘hei zhong cao’ (in China), and ‘kalonji’ (in India). It is a popular spice widely distributed in Asian countries like India, Pakistan, Bangladesh, Afghanistan and Sri Lanka [15, 16]. Both, the seeds and oil from Nigella sativa can be used for medicinal purposes, and a few of them include anti-cancer, anti-diabetic, anti-hypertensive, anti-inflammatory, anti-microbial, analgesic, immunomodulatory, spasmolytic, gastro-protective, hepato-protective, renal-protective, bronchodilatory and anti-oxidant activities[17-20]. Among all the activities it showed spectacular hypoglycemic effect by various mechanisms, which is essential for the management of Diabetes mellitus in the affected population [21], and also it is imperative to know about the side effects of the drug being used for therapy. The current study explored the beneficial effects and emphasized mechanistic values of N. sativa on glycemic control based on the pre-clinical and clinical effects.
Methods
We retrieved the data from various known databases like Pubmed, Scopus, Google scholar and SciFinder. The search filter was kept for up to 10 years. To gather the information key words such as ‘thymoquinone’, ‘black cumin’, ‘Nigella sativa compound’, pharmacological activity of Nigella sativa, therapeutic potential of Nigella sativa, ‘anti-diabetic effect of Nigella sativa’ ‘effect of Nigella sativa on blood glucose levels’, ‘Nigella sativa in diabetes’, ‘toxic effects of Nigella sativa’. The search language of publications and articles was done in English.
Inclusion criteria
Articles conveying information about anti diabetic effect of Nigella sativa have been included in the study.
Investigatory aspects in pre-clinical and clinical studies
Nigella sativa as an anti-diabetic drug on pre-clinical platform
Many studies have been carried out on the potential effects of Nigella sativa on diabetes mellitus and were found that it reduced blood glucose levels, NO (Nitrous oxide), HbA1c and altered lipid profile [22]. Studies were conducted on rats and mice using the streptozotocin-induced diabetic model and explored the mechanisms as described below [23]. Partial regeneration of pancreatic beta cells with the secretion of insulin was also observed with the N. sativa [24]. Previous studies revealed that the presence of thymoquinone compound in Nigella sativa is responsible for its anti-diabetic effect, also N. sativa acts on AMPK (AMP-activated protein kinase), thereby inhibiting gluconeogenesis in both the liver and muscles, aids in decreased absorption of glucose from the intestine [25, 26]. The duration of studies, doses and routes of administration along with the standard drug and the test drugs, were depicted in table 1.
Table 1: Effect of Nigella sativa on experimental diabetes in animal models
| S. No. | Models for induction of diabetes mellitus in animals | Dose and duration | Effect of N. sativa/Thymoquinone on blood glucose | References |
| 1 | STZ induced diabetes (60 mg/kg,ip) in rats |
N. sativa extract (200 and 400 mg/kg oral route) 6 w | Serum glucose levels decrease significantly | [27] |
| 2 | STZ induced diabetes (90 mg/kg, i. p) in rats. | Methanolic extract of N. sativa (25,50,100 and 200 μg/ml in situ intestinal perfusion technique) 3 mo | Enhanced glucose utilization and decrease glucose absorption from GIT. Improved insulin release from beta cells in rats (thereby effective in lowering serum glucose levels) |
[28] |
| 3 | STZ induced diabetes (30 mg/kg body weight i. p) in rats | N. sativa 0.5 ml, 1 ml, 1.5 ml per rat was administered orally. 40 d | Significant anti-diabetic effect with three doses due to the regeneration of beta cells of pancreas. | [23] |
| 4 | Nicotinamide (110 mg/kg) and Streptozotocin (65 mg/kg, i. p) induced in rats | Thymoquinone (20, 40, 80 mg/kg,p. o.) 21 d |
Decreases fasting blood sugar levels effectively. Decreases gluconeogenesis in liver. Increases utilization of glucose by increased sensitivity to the release of insulin from pancreas. | [29, 30] |
| 5 | STZ induced in rats (65 mg/kg body weight) |
Thymoquinone (at a dose of 50 mg/kg body weight) by gastric lavage 4 w | Significant decrease in the levels of HbA1c, lipid peroxidase and NO (Nitric Oxide) | [22] |
| 6 | STZ induced in rats (30 mg/kg bodyweight) |
Nigella sativa seed extract (0,24,48,72 mg/kg body weight) orally 4 w | Prevents polyphagia, weight loss and improves blood glucose levels in type diabetic rats. | [31] |
| 7 | STZ induced in rats (40 mg/kg body weight,i. p.) |
Thymoquinone (10,20 mg/kg, orally) 14 w |
Significant reduction in blood glucose levels, additionally lipid profile and PPARγ levels were improved. | [22] |
| 8 | STZ (150 mg/kg,i. p.), induced In mice | Metformin+Thymoquinone (200 mg/kg+50 mg/kg) orally 21 d | Showed a distinct hypoglycemic effects along with metformin. | [33] |
| 9 | Alloxan induced (150 mg/kg) in rabbits | N. sativa oil 2.5 ml/kg body weight orally 24 d | Found to be effective in reducing blood glucose levels | [34] |
| 10 | STZ (50 mg/kg body weight, i. p.) induced in rats | Thymoquinone (20 mg/kg/day by gavage) 5 w | Serum glucose levels decrease | [18, 35] |
| 11 | In vitro biochemical assay | N. sativa silver nano particles | Inhibits alpha amylase activity | [36] |
Thymoquinone and Nigella sativa extract was found to produce synergistic action on blood glucose levels with standard anti diabetic drugs like metformin [37] and glibenclimide [38].
Clinical aspects of N. sativa
With the administration of N. sativa clinically, few reports revealed that this plant was effective against hyperglycemia and hyperlipidemia [39]. Patients given with N. sativa seeds, extracts and oil were found to have a reduction in fasting blood glucose levels (FBG), post prandial blood glucose (PPBG) levels with improvement in glycated hemoglobin (HbA1c) levels, decreased triglycerides and increased high density lipoproteins (HDL) [40, 41]. The investigation included a maximum of 113 patients to evaluate the anti-diabetic clinical trials [42]. In almost all investigations, N. sativa was evaluated with the co-administration of any conventional drug, thus might be help analyze the synergistic effect, with no adverse effects [43].
Probable mechanism of action of N. sativa
Nigella sativa acts as an anti-hyperglycemic agent by the various expected mechanisms from the mentioned data, thus binds to the insulin receptors in the pancreas, increases glucose uptake by the cells, activates voltage-sensitive calcium channels and binds to Peroxisome Proliferator Activated Receptor gamma (PPAR-γ) in the nucleus. In contrast to this drug, conventional hypoglycemic drugs either bind to receptors (or) only act on glucose metabolism to exert their mechanism whereas NS has multiple hypoglycemic mechanisms to reduce blood sugar levels.
NS inhibits gluconeogenesis in the liver by releasing insulin from secretagogues, it activates insulin receptors, which enhances the production of cAMP and also cause calcium-dependent depolarization of cell concomitantly blocking ATP-sensitive k+ channels. NS affects glucose metabolism through GLUT-2 transporter, which will result in decrease number of glycated hemoglobin (HbA1c) (fig. 2). NS also consequence in genetic transcription by binding to PPAR-γ in the nucleus, this mechanism equally plays a crucial role in inhibiting gluconeogenesis.
Table 2: Clinical aspects of Nigella sativa
| S. No. | Study design | Drug dose and duration | Sample size (n) | Effects | References |
| 1 | Randomized placebo-control | 30 ml Nigellasativa oil for 80 d | 41 | Fall in fasting blood glucose (FBG) levels and an increase in insulin levels. | [44, 45] |
| 2 | Perspective study | 500 mg NS seeds for 17 mo | 80 | Significant decrease in fasting and postprandial blood glucose and improved HbA1c levels. | [40, 46] |
| 3 | Randomized clinical trial | 2.5 ml of NS seed oil for 3 mo | 70 | Improved HbA1c, decreased FBG and Post-prandial blood glucose (PPBG) | [41] |
| 4 | Randomized single blind control trial | 1.5-and 3-ml NS seed oil per day for 20 d | 99 | Significant decrease in HbA1c levels | [47] |
| 5 | Randomized double blind control trial | 3g/day NS oil soft capsules for 12 w | 72 | FBG, HbA1c, Triglycerides (TGs) and Basal metabolic index (BMI) changes. | [48] |
| 6 | Randomized control trial | 500 mg/kg NS seeds per day for 12 mo | 113 | Improved blood glucose levels and enhanced anti-oxidant system. | [42] |
| 7 | Randomized, double blind and placebo-controlled trial | 1 g of NS oil per day for 8 w | 44 | Improved HDL levels decreased FBG, liver enzymes and inflammatory mediators. in Non-Alcoholic Fatty Liver Disease (NAFLD) patients | [49-51] |
| 8 | Perspective, open-label randomized clinical trial | 450 mg NS oil capsule 3 times a day for 12 w | 44 | Significant decrease in serum levels of FBG. | [52] |
| 9 | Randomized clinical trial | 50 mg of TQ with 1000 mg of metformin for 90 d | 60 | Great reduction in FBG and PPBG was observed and improved HbA1c. | [31] |
| 10. | Randomized trial | 2 g of NS seeds crushed per day for 8 w | 40 | Marked effects on serum glucose levels and insulin were seen. | [53, 54] |
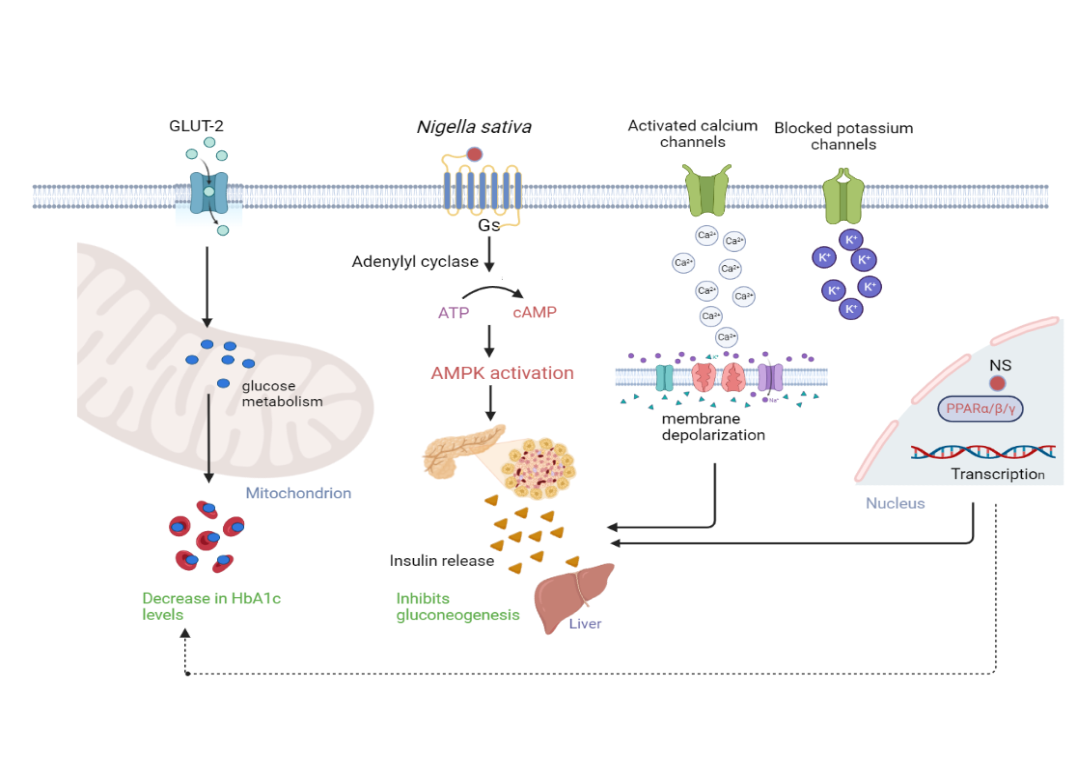
Fig. 2: Exploration of probable mechanistic insights of N. sativa at molecular basis. The mechanism was modified accordingly and created by using biorender software) [16, 18]
Adverse effects/Toxicological investigations of N. sativa
Based on the adverse effects of N. sativa, toxicological investigations were done by Abukhader et al. on possible adverse effects of thymoquinone in Wistar rats [55, 56]. Various adverse effects with different routes of administration, acute pancreatitis were observed in rats with i.p. injection and some short-term toxic effects were seen with oral administration in rats [57, 58]. Deaths were observed at 500 mg/kg dose [59]. Few studies have reported adverse effects like abnormal vision, dizziness and drowsiness [60], decreased BP and tachycardia [61], nausea, vomiting, stomachache, flatulence [62] and hypersensitivity reactions [63]. The exact clinical and preclinical doses which produce these effects were unknown [64]. A few effects of Thymoquinone were depicted on various organs in table 3.
Table 3: Side effects of N. sativa on experimental animals
| S. No. | Organs | NS side effects | References |
| 1. | Central Nervous System | Dizziness, drowsiness, fatigue, abnormal vision | [59] |
| 2. | Cardiovascular System | Tachycardia and hypotension | [60, 65] |
| 3. | Gastrointestinal System | Nausea, vomiting, flatulence, epigastric pain, abdominal cramps, diarrhea or constipation | [61, 17] |
| 4. | Renal system | Reversible nephritis, Crystalluria | [66] |
| 5. | Joints and muscles | Chondrotoxicity | [67] |
| 6. | others | Hypersensitivity reactions | [63] |
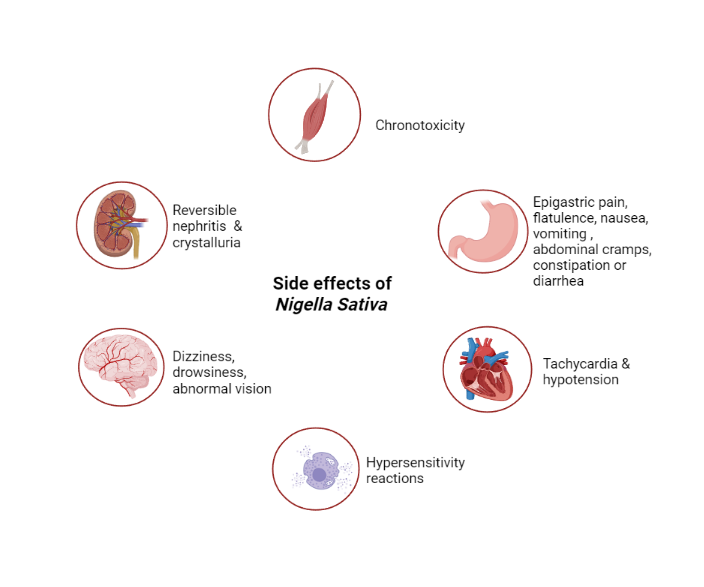
Fig. 3: Exploration of side effects of Nigella sativa, modified and changed accordingly using biorender software) [68]
CONCLUSION
It can be concluded that Nigella sativa exhibited an anti-hyperglycemic effect due to the presence of its chemical constituent, Thymoquinone. The mechanism was derived keeping given the above studies, however until present, in the clinical trials reported, the exact dose was in chaos, which might be considered as one of the causes for side effects. Thus, a mandate investigation on a large population becomes crucial for fixing the dose so that the side/adverse effects can be closely monitored and resolved simultaneously. Detecting a therapeutic dose, there inclines to favorable outcomes along with patient compliance. In the present review, though the mechanism was acquired, still significant, satisfactory and more detailed pharmacodynamics is possible with further studies. Additionally, as consumption of the conventional drug with the herbal medicines as a combination has been recommended in the present era, there is an option for a possible synergistic effect to be identified if exists. Hence, in future, experimental investigations on determination of the therapeutic dose of N. sativa are essential to elucidate the exact mechanism at the molecular level, which in turn may be helpful to conquer the side effects.
ACKNOWLEDGEMENT
Not applicable
AUTHORS CONTRIBUTIONS
SB has written the manuscript, guided and edited by SR, has also contributed in preparation of diagrammatic illustration in the manuscript. RKV and SB collected the information required for writing Manuscript.
CONFLICTS OF INTERESTS
The authors declared no conflicts of interest.
REFERENCES
Marpaung J, Siregar MF, Sitepu M, Bachtiar A. Black cumin (Nigella sativa) effect on blood pressure, mean arterial pressure proteinuria in preeclamptic model rats. Int J Curr Pharm Sci. 2020;12(4):127-33. doi: 10.22159/ijcpr.2020v12i4.39099.
Zulfikri Z, Harahap U, Ichwan M. Effect of black cumin oil (Nigella sativa L.) on spatial memory of adult mice treated with temozolomide. Asian J Pharm Clin Res. 2018;11(13):151-4. doi: 10.22159/ajpcr.2018.v11s1.26594.
World Health Organization. Global health estimates: deaths by cause age sex and country, 2000-2015. Geneva: World Health Organization; 2014.
Mendis S, Davis S, Norrving B. Organizational update: the World Health Organization global status report on noncommunicable diseases 2014; one more landmark step in the combat against stroke and vascular disease. Stroke. 2015;46(5):e121-2. doi: 10.1161/STROKEAHA.115.008097, PMID 25873596.
International Diabetes Federation IDF Diabetes Atlas. 10th ed. Brussels, Belgium: International Diabetes Federation; 2021.
Gur FM, Aktas I. The ameliorative effects of thymoquinone and beta-aminoisobutyric acid on streptozotocin-induced diabetic cardiomyopathy. Tissue Cell. 2021 Aug;71:101582. doi: 10.1016/j.tice.2021.101582, PMID 34171519.
Rao PS, Mohan GK. In vitro alpha-amylase inhibition and in vivo antioxidant potential of Momordica dioica seeds in streptozotocin induced oxidative stress in diabetic rats. Saudi J Biol Sci. 2017 Sep;24(6):1262-7. doi: 10.1016/j.sjbs.2016.01.010, PMID 28855820, PMCID PMC5562449.
Kumar R, Rao PS. Streptozotocin induced oxidative stress in diabetic rats a defensive effect of Psydrax dicoccos. Asian J Pharm Clin Res. 2018 Nov 7;11(11):378-80. doi: 10.22159/ajpcr.2018.v11i11.28019.
Ahmad MF, Ahmad FA, Ashraf SA, Saad HH, Wahab S, Khan MI. An updated knowledge of Black seed (Nigella sativa Linn.): review of phytochemical constituents and pharmacological properties. J Herb Med. 2021 Feb;25:100404. doi: 10.1016/j.hermed.2020.100404, PMID 32983848, PMCID PMC7501064.
Haseena S, Aithal M, Das KK, Saheb SH. Phytochemical analysis of Nigella sativa and its effect on reproductive system. J Pharm Sci Res. 2015;7(8):514-7.
Ahmad MF, Ahmad FA, Ashraf SA, Saad HH, Wahab S, Khan MI. An updated knowledge of black seed (Nigella sativa Linn.): review of phytochemical constituents and pharmacological properties. J Herb Med. 2021 Feb;25:100404. doi: 10.1016/j.hermed.2020.100404, PMID 32983848, PMCID PMC7501064.
Khader M, Eckl PM. Thymoquinone: an emerging natural drug with a wide range of medical applications. Iran J Basic Med Sci. 2014;17(12):950-7. PMID 25859298.
Malik S, Singh A, Negi P, Kapoor VK. Thymoquinone: a small molecule from nature with high therapeutic potential. Drug Discov Today. 2021;26(11):2716-25. doi: 10.1016/j.drudis.2021.07.013, PMID 34303824.
Farooq J, Sultana R, Taj T, Asdaq SM, Alsalman AJ, Mohaini MA. Insights into the protective effects of thymoquinone against toxicities induced by chemotherapeutic agents. Molecules. 2021 Dec 30;27(1):226. doi: 10.3390/molecules27010226, PMID 35011457, PMCID PMC8746502.
Khan MA. Thymoquinone a constituent of prophetic medicine, black seed is a miracle therapeutic molecule against multiple diseases. Int J Health Sci (Qassim). 2019;13(1):1-2. PMID 30842710.
Al Attass SA, Zahran FM, Turkistany SA. Nigella sativa and its active constituent thymoquinone in oral health. Saudi Med J. 2016;37(3):235-44. doi: 10.15537/smj.2016.3.13006, PMID 26905343.
Hannan MA, Rahman MA, Sohag AA, Uddin MJ, Dash R, Sikder MH, Tahjib Ul Arif M, Mitra S, Oktaviani DF, Khan MK, Choi HJ, Moon IS, Kim B. Black cumin (Nigella sativa L.): a comprehensive review on phytochemistry health benefits molecular pharmacology and safety. Nutrients. 2021 May 24;13(6):1784. doi: 10.3390/nu13061784, PMID 34073784.
Aktas I, Mehmet Gur F. Hepato protective effects of thymoquinone and beta-aminoisobutyric acid in streptozocin induced diabetic rats. Biotech Histochem. 2022;97(1):67-76. doi: 10.1080/10520295.2021.1949041, PMID 34281431.
Asaduzzaman Khan M, Tania M, Fu S, Fu J. Thymoquinone as an anticancer molecule: from basic research to clinical investigation. Oncotarget. 2017;8(31):51907-19. doi: 10.18632/oncotarget.17206, PMID 28881699.
Goyal SN, Prajapati CP, Gore PR, Patil CR, Mahajan UB, Sharma C. Therapeutic potential and pharmaceutical development of thymoquinone: a multitargeted molecule of natural origin. Front Pharmacol. 2017 Sep 21;8:656. doi: 10.3389/fphar.2017.00656, PMID 28983249.
Agarwal S, Tripathi S, Arshi A, Mishra N. Nutritional composition and antioxidant profiles of Nigella sativa L. seeds. AP. 2020;9(2):207-14. doi: 10.21276/ap.2020.9.2.18.
Faisal Lutfi M, Abdel Moneim AH, Alsharidah AS, Mobark MA, Abdellatif AA, Saleem IY. Thymoquinone lowers blood glucose and reduces oxidative stress in a rat model of diabetes. Molecules. 2021;26(8):2348. doi: 10.3390/molecules26082348, PMID 33920728.
Sadiq N, Subhani G, Fatima SA, Nadeem M, Zafer S, Mohsin M. Antidiabetic effect of Nigella sativa compared with metformin on blood glucose levels in streptozotocin induced diabetic albino Wistar rats. Int J Basic Clin Pharmacol. 2021;10(4):361-7. doi: 10.18203/2319-2003.ijbcp20211016.
Jangjo Borazjani S, Dastgheib M, Kiyamarsi E, Jamshidi R, Rahmati Ahmadabad S, Helalizadeh M. Effects of resistance training and Nigella sativa on type 2 diabetes: implications for metabolic markers low grade inflammation and liver enzyme production. Arch Physiol Biochem. 2023 Dec;129(4):913-21. doi: 10.1080/13813455.2021.1886117, PMID 33612031.
Coughlan KA, Valentine RJ, Ruderman NB, Saha AK. AMPK activation: a therapeutic target for type 2 diabetes? Diabetes Metab Syndr Obes. 2014 Jun 24;7:241-53. doi: 10.2147/DMSO.S43731, PMID 25018645.
Dalli M, Daoudi NE, Azizi SE, Benouda H, Bnouham M, Gseyra N. Chemical composition analysis using HPLC-UV/GC-MS and inhibitory activity of different Nigella sativa fractions on pancreatic α-amylase and intestinal glucose absorption. BioMed Res Int. 2021 Jun 26;2021:9979419. doi: 10.1155/2021/9979419, PMID 34258287, PMCID PMC8257330.
Mohebbati R, Abbasnezhad A, Havakhah S, Mousavi M. The effect of Nigella sativa on renal oxidative injury in diabetic rats. Saudi J Kidney Dis Transpl. 2020;31(4):775-86. doi: 10.4103/1319-2442.292311, PMID 32801238.
Hannan JM, Ansari P, Haque A, Sanju A, Huzaifa A, Rahman A. Nigella sativa stimulates insulin secretion from isolated rat islets and inhibits the digestion and absorption of (CH2O)n in the gut. Biosci Rep. 2019;39(8):BSR20190723. doi: 10.1042/BSR20190723.
Rani R, Dahiya S, Dhingra D, Dilbaghi N, Kim KH, Kumar S. Improvement of antihyperglycemic activity of nano-thymoquinone in rat model of type 2 diabetes. Chem Biol Interact. 2018 Nov 1;295:119-32. doi: 10.1016/j.cbi.2018.02.006, PMID 29421519.
Ramineedu K, Sankaran KR, Mallepogu V, Rendedula DP, Gunturu R, Gandham S. Thymoquinone mitigates obesity and diabetic parameters through regulation of major adipokines key lipid metabolizing enzymes and AMPK/p-AMPK in diet-induced obese rats. 3 Biotech. 2024 Jan;14(1):16. doi: 10.1007/s13205-023-03847-x, PMID 38125651, PMCID PMC10728404.
Susilowati R, Ainuzzakki V, Nadif MR, Diana AR. The efficacy of Nigella sativa L. extracts to reduce cardiovascular disease risk in diabetic dyslipidemia. AIP Conf Proc. 2019;2120(1):700020. doi: 10.1063/1.5115737.
Alshahrani S, Anwer T, Alam MF, Ahmed RA, Khan G, Sivakumar SM. Effect of thymoquinone on high fat diet and STZ-induced experimental type 2 diabetes: a mechanistic insight by in vivo and in silico studies. J Food Biochem. 2021;45(8):e13807. doi: 10.1111/jfbc.13807, PMID 34152002.
Dalli M, Bekkouch O, Azizi SE, Azghar A, Gseyra N, Kim B. Nigella sativa L. phytochemistry and pharmacological activities: a review (2019-2021). Biomolecules. 2021;12(1):20. doi: 10.3390/biom12010020, PMID 35053168.
Akhtar MT, Qadir R, Bukhari I, Ashraf RA, Malik Z, Zahoor S. Antidiabetic potential of Nigella sativa L. seed oil in alloxaninduced diabetic rabbits. Trop J Pharm Res. 2020;19(2):283-9. doi: 10.4314/tjpr.v19i2.10.
Gur FM, Aktas I. The effects of thymoquinone and β-aminoisobutyric acid on brain tissue of streptozotocin induced diabetic rats. Int J Vet Ani Res. 2021;4(1):1-6.
RP, Anitha R, SR, TL. Anti-diabetic activity of silver nanoparticles prepared from cumin oil using alpha amylase inhibitory assay. IJRPS. 2020;11(2):1267-9. doi: 10.26452/ijrps.v11i2.1978.
Ali SM, Chen P, Sheikh S, Ahmad A, Ahmad M, Paithankar M. Thymoquinone with metformin decreases fasting post prandial glucose and HbA1c in type 2 diabetic patients. Drug Res (Stuttg). 2021;71(6):302-6. doi: 10.1055/a-1388-5415, PMID 33684953.
Ahmad A, Khan RM, Alkharfy KM, Raish M, Al Jenoobi FI, Al Mohizea AM. Effects of thymoquinone on the pharmacokinetics and pharmacodynamics of glibenclamide in a rat model. Nat Prod Commun. 2015;10(8):1395-8. doi: 10.1177/1934578X1501000821, PMID 26434126.
Heshmati J, Namazi N. Effects of black seed (Nigella sativa) on metabolic parameters in diabetes mellitus: a systematic review. Complement Ther Med. 2015;23(2):275-82. doi: 10.1016/j.ctim.2015.01.013, PMID 25847566.
Wei J, Wang B, Chen Y, Wang Q, Ahmed AF, Cui L. Effects of two triterpenoids from Nigella sativa seeds on insulin resistance of 3T3-L1 adipocytes. Front Nutr. 2022 Aug 23;9:995550. doi: 10.3389/fnut.2022.995550, PMID 36082026, PMCID PMC9445806.
Hosseini M, Mirkarimi S, Amini M, Mohtashami R, Kianbakht S, Fallah HH. Effects of Nigella sativa L. seed oil in type II diabetic patients: a randomized double-blind placebo controlled clinical trial. J Med Plants. 2013;12(47):93-9.
Kaatabi H, Bamosa AO, Badar A, Al Elq A, Abou Hozaifa B, Lebda F. Nigella sativa improves glycemic control and ameliorates oxidative stress in patients with type 2 diabetes mellitus: placebo controlled participant blinded clinical trial. PLOS One. 2015;10(2):e0113486. doi: 10.1371/journal.pone.0113486, PMID 25706772.
Hamdan A, Haji Idrus R, Mokhtar MH. Effects of Nigella sativa on type 2 diabetes mellitus: a systematic review. Int J Environ Res Public Health. 2019;16(24):4911. doi: 10.3390/ijerph16244911, PMID 31817324.
Talebi A, Maham M, Asri Rezaei S, Pournaghi P, Khorrami MS, Derakhshan A. Effects of Nigella sativa on performance blood profiles and antibody titer against Newcastle disease in broilers. Evid Based Complement Alternat Med. 2021 Jun 14;2021:2070375. doi: 10.1155/2021/2070375, PMID 34234833, PMCID PMC8216824.
Tavakkoli A, Mahdian V, Razavi BM, Hosseinzadeh H. Review on clinical trials of black seed (Nigella sativa) and its active constituent thymoquinone. J Pharmacopuncture. 2017;20(3):179-93. doi: 10.3831/KPI.2017.20.021, PMID 30087794.
Mahomoodally MF, Aumeeruddy MZ, Legoabe LJ, Montesano D, Zengin G. Nigella sativa L. and its active compound thymoquinone in the clinical management of diabetes: a systematic review. Int J Mol Sci. 2022;23(20):12111. doi: 10.3390/ijms232012111, PMID 36292966.
Rachman PN, Akrom EA, Darmawan E. The efficacy of black cumin seed (Nigella sativa) oil and hypoglycemic drug combination to reduce HbA1c level in patients with metabolic syndrome risk. IOP Conf Ser.: Mater Sci Eng. 2017;259:12018. doi: 10.1088/1757-899X/259/1/012018.
Heshmati J, Namazi N, Memarzadeh MR, Taghizadeh M, Kolahdooz F. Nigella sativa oil affects glucose metabolism and lipid concentrations in patients with type 2 diabetes: a randomized double-blind placebo controlled trial. Food Res Int. 2015;70:87-93. doi: 10.1016/j.foodres.2015.01.030.
Rashidmayvan M, Mohammadshahi M, Seyedian SS, Haghighizadeh MH. The effect of Nigella sativa oil on serum levels of inflammatory markers liver enzymes lipid profile insulin and fasting blood sugar in patients with non-alcoholic fatty liver. J Diabetes Metab Disord. 2019;18(2):453-9. doi: 10.1007/s40200-019-00439-6, PMID 31890671.
Tang G, Zhang L, Tao J, Wei Z. Effect of Nigella sativa in the treatment of nonalcoholic fatty liver disease: a systematic review and meta-analysis of randomized controlled trials. Phytother Res. 2021;35(8):4183-93. doi: 10.1002/ptr.7080, PMID 33728708.
Heshmati J, Namazi N, Memarzadeh MR, Taghizadeh M, Kolahdooz F. Nigella sativa oil affects glucose metabolism and lipid concentrations in patients with type 2 diabetes: a randomized double-blind, placebo-controlled trial. Food Res Int. 2015;70:87-93. doi: 10.1016/j.foodres.2015.01.030.
Moustafa HA, El Wakeel LM, Halawa MR, Sabri NA, El Bahy AZ, Singab AN. Effect of Nigella sativa oil versus metformin on glycemic control and biochemical parameters of newly diagnosed type 2 diabetes mellitus patients. Endocrine. 2019;65(2):286-94. doi: 10.1007/s12020-019-01963-4, PMID 31152309.
Jangjo Borazjani S, Dastgheib M, Kiyamarsi E, Jamshidi R, Rahmati Ahmadabad S, Helalizadeh M. Effects of resistance training and nigella sativa on type 2 diabetes: implications for metabolic markers low-grade inflammation and liver enzyme production. Arch Physiol Biochem. 2023;129(4):913-21. doi: 10.1080/13813455.2021.1886117, PMID 33612031.
Sahebkar A, Beccuti G, Simental Mendia LE, Nobili V, Bo S. Nigella sativa (black seed) effects on plasma lipid concentrations in humans: a systematic review and meta-analysis of randomized placebo-controlled trials. Pharmacol Res. 2016;106:37-50. doi: 10.1016/j.phrs.2016.02.008, PMID 26875640.
Zielinska M, Deren K, Polak Szczybylo E, Stępien AE. The role of bioactive compounds of Nigella sativa in rheumatoid arthritis therapy current reports. Nutrients. 2021;13(10):3369. doi: 10.3390/nu13103369, PMID 34684370.
Maideen NM. Antidiabetic activity of Nigella sativa (Black Seeds) and its active constituent (thymoquinone): a review of human and experimental animal studies. Chonnam Med J. 2021;57(3):169-75. doi: 10.4068/cmj.2021.57.3.169, PMID 34621636.
Alam M, Hasan GM, Ansari MM, Sharma R, Yadav DK, Hassan MI. Therapeutic implications and clinical manifestations of thymoquinone. Phytochemistry. 2022 Aug;200:113213. doi: 10.1016/j.phytochem.2022.113213, PMID 35472482.
Adana MY, Imam A, Bello AA, Sunmonu OE, Alege EP, Onigbolabi OG. Oral thymoquinone modulates cyclophosphamide induced testicular toxicity in adolescent Wistar rats. Andrologia. 2022 May;54(4):e14368. doi: 10.1111/and.14368, PMID 34997774.
Hannan MA, Rahman MA, Sohag AA, Uddin MJ, Dash R, Sikder MH, Tahjib-Ul-Arif M, Mitra S, Oktaviani DF, Khan MK, Choi HJ, Moon IS, Kim B. Black Cumin (Nigella sativa L.): a comprehensive review on phytochemistry health benefits molecular pharmacology and safety. Nutrients. 2021 May 24;13(6):1784. doi: 10.3390/nu13061784.
Farkhondeh T, Samarghandian S, Hozeifi S, Azimi Nezhad M. Therapeutic effects of thymoquinone for the treatment of central nervous system tumors: a review. Biomed Pharmacother. 2017 Dec;96:1440-4. doi: 10.1016/j.biopha.2017.12.013, PMID 29223556.
Liu H, Liu HY, Jiang YN, Li N. Protective effect of thymoquinone improves cardiovascular function and attenuates oxidative stress inflammation and apoptosis by mediating the PI3K/Akt pathway in diabetic rats. Mol Med Rep. 2016;13(3):2836-42. doi: 10.3892/mmr.2016.4823, PMID 26820252.
Shakeri F, Gholamnezhad Z, Megarbane B, Rezaee R, Boskabady MH. Gastrointestinal effects of Nigella sativa and its main constituent thymoquinone: a review. Avicenna J Phytomed. 2016;6(1):9-20. PMID 27247918.
Al Qubaisi MS, Rasedee A, Flaifel MH, Eid EE, Hussein Al Ali S, Alhassan FH. Characterization of thymoquinone/hydroxypropyl-β-cyclodextrin inclusion complex: application to anti-allergy properties. Eur J Pharm Sci. 2019;133:167-82. doi: 10.1016/j.ejps.2019.03.015, PMID 30902654.
Hossain MS, Sharfaraz A, Dutta A, Ahsan A, Masud MA, Ahmed IA. A review of ethnobotany phytochemistry antimicrobial pharmacology and toxicology of Nigella sativa L. Biomed Pharmacother. 2021 Nov;143:112182. doi: 10.1016/j.biopha.2021.112182, PMID 34649338.
Farkhondeh T, Samarghandian S, Shahri AM, Samini F. The neuroprotective effects of thymoquinone: a review. Dose Response. 2018;16(2):1559325818761455. doi: 10.1177/1559325818761455, PMID 29662431.
Zhu N, Xiang Y, Zhao X, Cai C, Chen H, Jiang WB. Thymoquinone suppresses platelet derived growth factor-BB-induced vascular smooth muscle cell proliferation migration and neointimal formation. J Cell Mol Med. 2019;23(12):8482-92. doi: 10.1111/jcmm.14738, PMID 31638340.
Liou YF, Hsieh YS, Hung TW, Chen PN, Chang YZ, Kao SH. Thymoquinone inhibits metastasis of renal cell carcinoma cell 786-O-SI3 associating with downregulation of MMP-2 and u-PA and suppression of PI3K/Src signaling. Int J Med Sci. 2019;16(5):686-95. doi: 10.7150/ijms.32763, PMID 31217736.
Chopra H, Bibi S, Singh I, Kamal MA, Islam F, Alhumaydhi FA. Nanomedicines in the management of Alzheimer’s disease: current view and future prospects. Front Aging Neurosci. 2022;14:879114. doi: 10.3389/fnagi.2022.879114, PMID 35875806.