
Int J Pharm Pharm Sci, Vol 17, Issue 9,28-35Original Article
QUALITATIVE AND QUANTITATIVE ANALYSIS OF FLAVONOIDS, TERPENOIDS, AND POLYPHENOLS IN CHAMOMILE FLOWERS BY HPLC AND GC-MS METHODS
RAJA KUMAR PARABATHINA*, NIDHI NILESH DUBEY, SANIKA SWAPNIL GIRGAONKAR, VISHAL NANDAKUMAR LOLGE, KUSHAL KONDBA NARWADE
Department of Biochemistry, Institute of Biosciences and Technology, MGM University, Chh. Sambhajinagar-431003, India
*Corresponding author: Raja Kumar Parabathina; *Email: rajakumar.parabathina@gmail.com
Received: 11 May 2025, Revised and Accepted: 07 Jul 2025
ABSTRACT
Objective: The current study aimed to investigate the bioactive compounds, including flavonoids, terpenoids, and polyphenols, present in Matricaria chamomilla (chamomile) flowers using HPLC and GC-MS methods.
Methods: HPLC and GC-MS methods were employed for the estimation of flavonoids, terpenoids, and polyphenols in chamomile flower extract, with standard procedures, and an RP-18 column was employed for HPLC. Whereas the GC-MS was conducted at Toshvin Research and Application Centre (TRACe), Mumbai, using a Shimadzu GC-MS-TQ8050NX system with an AOC-30 autosampler.
Results: The total phenolic content (TPC) of the hydro-alcoholic extract is found to be 782.1 mg GAE/g, indicating a high concentration of antioxidant compounds. The DPPH assay further confirmed this, showing a 75.1% radical scavenging activity at 10 µl** concentration. High-performance liquid chromatography (HPLC) analysis identified apigenin as the predominant flavonoid, with the highest peak area (44.432), followed by Morin, Naringin, Quercetin, and Rutin, suggesting it as a key compound contributing to chamomile’s medicinal properties. Gas chromatography-mass spectrometry (GC-MS) analysis of the n-hexane extract led to the identification of 20 compounds. These included a variety of alkanes, terpenoids (such as phytol and caryophyllene oxide), sterols (e. g., stigmasterol, ergost-5-en-3-ol), fatty acid esters, siloxanes, and other hydrocarbons. Several compounds, including squalene, phytol, and erucamide, have been studied for their established anticancer and antimicrobial activities.
Conclusion: These results affirm the phytochemical richness of chamomile and support its continued exploration as a source of natural compounds for pharmaceutical and therapeutic applications.
Keywords: Matricaria chamomilla, Phytochemicals, HPLC, GC-MS, Apigenin
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijpps.2025v17i9.54977 Journal homepage: https://innovareacademics.in/journals/index.php/ijpps
INTRODUCTION
Chamomile is a well-known medicinal herb belonging to the Asteraceae family and has been used for centuries in Chinese traditional medicine. It is native to Europe and was introduced to India 300 years ago. There are two main varieties of chamomile: Matricaria chamomilla L. (MC) and Chamaemelumnobile (CN). Among these, MC has been extensively researched and is proven to be non-toxic to both animals and humans, making it widely used [1].
Though chamomile is primarily known for its use in traditional medicines, it is also consumed as tea and as a vitamin supplement. The bioactive components in chamomile are responsible for its anti-inflammatory, antioxidant, and anticancer properties [2].
Chamomile flowers contain over 120 bioactive components. The essential oil contains non-phenolic compounds, primarily terpenoids such as α-bisabolol and its oxides, chamazulene, and β-farnesene. On the other hand, chamomile extracts, obtained using solvents or water, are rich in phenolic compounds, including flavonoids, coumarins, and some phenolic acids. The presence of these compounds makes chamomile an effective natural therapy, as it exhibits multiple pharmacological effects [3].
Terpenoids, also called isoprenoids, are a diverse class of naturally occurring organic compounds derived from five-carbon isoprene units (C₅H₈). They are present in fruits, flowers, trees, and spices. These compounds exhibit a wide range of structures and are associated have various biological activities, including antioxidant, antimicrobial, anti-inflammatory, antiallergic, anticancer, antimetastatic, and apoptosis induction. Due to these properties, terpenoids have potential applications in the food, cosmetics, pharmaceutical, and medical industries [4].
Flavonoids have a core structure of 2-phenylchromone. Around fifty flavonoids have been identified in chamomile, which are its main active components. These include Quercetin, Apigenin, Naringin, and Rutin. These compounds exhibit antibacterial, antioxidant, and other pharmacological effects. According to reports, Apigenin is the most abundant flavonoid and possesses anti-inflammatory and pro-apoptotic properties. It also prevents metastasis, thereby inhibiting tumor growth and ultimately exhibiting anticancer effects [1].
These compounds exhibit antimicrobial and anti-inflammatory activity. Umbelliferone exhibits biological properties such as antioxidant activity in vitro, inhibition of HIV-1 replication, and inhibition of cell proliferation of different human tumor cell lines. It is used in sunscreens as it strongly absorbs ultraviolet light at several wavelengths. Herniarin is also well known for its application in pharmaceutical and cosmetic products [5].
High-Performance Liquid Chromatography (HPLC) is a powerful technique used for the separation and quantification of phytochemicals based on their differential interaction with the stationary and mobile phases. In this study, HPLC is employed to analyse flavonoids and polyphenols in chamomile extract due to its high sensitivity and precision. The use of a gradient solvent system enhanced the resolution of compounds with varying polarities, improving analytical efficiency. HPLC remains one of the most reliable methods for profiling bioactive compounds in plant materials [6].
The current study focuses on the quality and quantitative estimation of three key bioactive compounds found in chamomile, such as Terpenoids, Flavonoids, and polyphenols, by HPLC and GC-MS.
MATERIALS AND METHODS
Chemicals and reagents
The following chemicals and reagents were used in the study: Ethanol (analytical grade) from Merck, India, was employed as a polar solvent in the hydro-alcoholic extraction process, while distilled water of laboratory grade was used for extraction and reagent preparation. n-Hexane (analytical grade), also from Merck, served as a non-polar solvent for extracting lipophilic compounds. Gallic acid (Sigma-Aldrich, USA) was used as a standard for estimating, Total Phenolic Content (TPC), and the Folin–Ciocalteu reagent (SRL Chemicals, India) was utilized in the colorimetric assay to detect phenolic compounds. Sodium carbonate (Na₂CO₃) from Merck was used to react with the Folin–Ciocalteu reagent.
Methanol (HPLC grade, Merck) was used for preparing the DPPH solution and sample dilution, while DPPH (2,2-diphenyl-1-picrylhydrazyl) obtained from Sigma-Aldrich, USA, was employed for the antioxidant activity assay. Magnesium turnings (Qualigens, India) were used in the Shinoda test to detect flavonoids. Concentrated hydrochloric acid (HCl), ferric chloride (FeCl₃), glacial acetic acid, concentrated sulfuric acid (H₂SO₄), 1N sodium hydroxide (NaOH), and chloroform (CHCl₃), all sourced from Merck or LobaChemie, were used for various phytochemical screening tests including detection of flavonoids, tannins, glycosides, terpenoids, and coumarins.
Acetonitrile (HPLC grade, Merck, India) served as the mobile phase in HPLC analysis, while formic acid (0.01%, Sigma-Aldrich, USA) was used as a modifier. Additionally, standard flavonoids such as apigenin, morin, naringin, quercetin, and rutin (Sigma-Aldrich, USA) were utilized for HPLC calibration and identification of flavonoid content in the samples.
Collection and preparation of chamomile flower
Chamomile flowers (German chamomile – CAS No. 84082-60-0-Matricaria recutita) free from pests and diseases, were collected from Jaywant Green Bliss Corporation, Talegaon (Pune). The flowers were shed, dried at room temperature for a week. Then they were finely ground using a mechanical mixer blender to achieve a uniform powder.
Extraction of chamomile flower bioactive compounds using the maceration method
The extraction of bioactive compounds from Matricaria chamomilla (chamomile) flowers was carried out by using the maceration method. Dried powder of chamomile flowers was subjected to solvent extraction using both polar and non-polar solvents to ensure a broad spectrum of compound isolation. For the extraction of polar compounds such as flavonoids, phenolic acids, and tannins, a hydro-alcoholic solvent system (ethanol: water in a 50:50 ratio) was used [8].
The 11g powdered flower was macerated with the hydro-alcoholic solvent in a conical flask at room temperature for 2-3 days. For the extraction of non-polar compounds, such as essential oils, terpenoids, and waxes, n-hexane is used as the solvent. The same procedure is followed where the powdered plant material was macerated with n-hexane at room temperature for 2-3 days. This dual-solvent maceration approach allowed the selective extraction of both phenolic and non-phenolic secondary metabolites. The extracts were then stored at room temperature until further use in the analytical assay. After maceration, the chamomile extract was filtered through muslin cloth to remove coarse plant debris, followed by fine filtration using Whatman No. 1 filter paper. For further purification, the concentrated extract was subjected to back extraction (liquid-liquid partitioning). The hydro-alcoholic extract was redissolved in n-hexane using a separating funnel. This technique facilitated the selective separation of bioactive constituents based on their polarity, aiding in the enrichment and isolation of specific phytochemical classes.
Quantitative analysis of total phenolic content by using a spectrophotometer
The total phenolic content (TPC) of the hydroalcoholic extract of chamomile was determined using the Folin–Ciocalteu colorimetric method. A standard stock solution of Gallic acid was prepared at a concentration of 0.02 mg/ml and further diluted to obtain a range of working standards. Aliquots ranging from 20 to 100 µl** of the diluted standards were mixed with Ethanol, followed by the addition of 250 µl** of diluted Folin–Ciocalteu reagent (1:10) and 1.5 ml of 7.5% sodium carbonate solution. The reaction mixtures were incubated for 30 min, and absorbance was recorded at 740 nm using a UV-Visible spectrophotometer. The TPC of the extract was quantified by extrapolating the absorbance values on a Gallic acid calibration curve and expressed as milligrams of Gallic acid equivalents per g of extract (mg GAE/g) [9].
Preliminary phytochemical screening of chamomile extract
Phytochemical tests were performed to ascertain the presence of bioactive compounds [10].
Test for flavonoids
To detect flavonoids, the Shinoda test was performed. 0.5 ml of the extract was mixed with magnesium turnings and a few drops of concentrated hydrochloric acid. The appearance of a pink, red, or orange coloration indicates the presence of flavonoids, due to the formation of flavonoid-metal complexes.
Test for tannins
About 0.5 ml of extract was dissolved in 20 ml of distilled water and boiled in a test tube, and filtered. To this filtrate, a few drops of 0.1% ferric chloride solution was added. Formation of a blackish blue colour indicated the presence of tannin.
Test for glycosides
About 1 ml of glacial acetic acid containing traces of ferric chloride and 1 ml of concentrated sulphuric acid was added to 0.5 ml of plant extracts (alcoholic extracts). Formation of reddish-brown color at the junction and its change to bluish green in the upper layer indicated the presence of glycosides.
Test for coumarins
A 0.5 ml of the extract was mixed with 1N sodium hydroxide and observed under UV light. The presence of coumarins was indicated by the appearance of blue or green fluorescence, due to their characteristic lactone structure that fluoresces under alkaline conditions.
Test for terpenoids
A 0.5 ml of n-hexane extract was dissolved in 2 ml of chloroform, and 3 ml of concentrated. H2SO4 was added along the wall of the test tube. The reddish-brown color in the interface indicates the presence of terpenoids.
Test for saponins
0.5 ml of extract was diluted in 20 ml of distilled water, and the suspension was shaken in the test tube. The development of a layer of foam indicates Saponins.
Evaluation of antioxidant activity of chamomile extract
The antioxidant activity of the extract was evaluated using the DPPH radical scavenging assay. A 0.024% DPPH solution was prepared by dissolving 2.4 mg of DPPH in 100 ml of methanol. For the reaction, 5 μL of the extract was added to 3.995 ml of the DPPH solution, mixed, and incubated in the dark at room temperature for 30 min. Absorbance was measured at 515 nm using a UV-Vis spectrophotometer. A blank without a sample was used as a control. The radical scavenging activity was calculated by using the given formula [11].
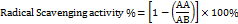
Analysis of hydro-alcoholic chamomile extract by using HPLC
The HPLC analysis of chamomile extract was performed using a gradient elution on a reverse-phase column with Solvent A (water: 0.01% formic acid) and Solvent B (acetonitrile). The 30-minute run began with 50% B, decreased to 15% at 5 min, and increased to 100% B by 27 min, returning to 50% by 30 min. The flow rate was 1.0 ml/min, the injection volume was 20 µl**, detection at 350 nm, and analysis was at room temperature. This gradient allowed efficient separation of compounds with varying polarity and improved resolution [6].
Analysis of n-hexane chamomile extract by using GC-MS
GC-MS analysis of n-hexane chamomile extract was done at Toshvin Research and Application Centre (TRACe), Mumbai, using a Shimadzu GC-MS-TQ8050NX system with an AOC-30 autosampler. A 100 mg sample of chamomile flower powder was extracted using 1 ml of n-hexane, vortexed, sonicated for 1 minute, and centrifuged at 5000 rpm for 10 min. The separation was performed on an SH-5 Sil MS capillary column (30 m × 0.25 mm × 0.25 μm). Helium was used as the carrier gas at a flow rate of 1.5 ml/min. The injection was split (1:10) with a volume of 0.5 µl**, and the injector was maintained at 280 °C. Oven temperature was programmed from 40 °C (2 min hold) to 150 °C at 10 °C/min (2 min), then to 250 °C at 15 °C/min (2 min), and finally to 300 °C at 25 °C/min (16 min hold). Mass spectrometry was operated in EI mode (35–700 m/z), with a source temperature of 230 °C and interface temperature of 280 °C. Compound identification was based on mass spectral matching with the NIST library.
RESULTS
Quantitative analysis of total phenolic content by using a spectrophotometer
The Total Phenolic Content (TPC) of the hydro-alcoholic extract of chamomile flowers is determined using the Folin-Ciocalteu method, with Gallic acid as the standard (fig. 1). Based on the standard calibration curve (y = 0.5279x – 0.0257) and accounting for a 1:100 dilution factor, the phenolic content is found to be 71.1 mg GAE/ml of extract.
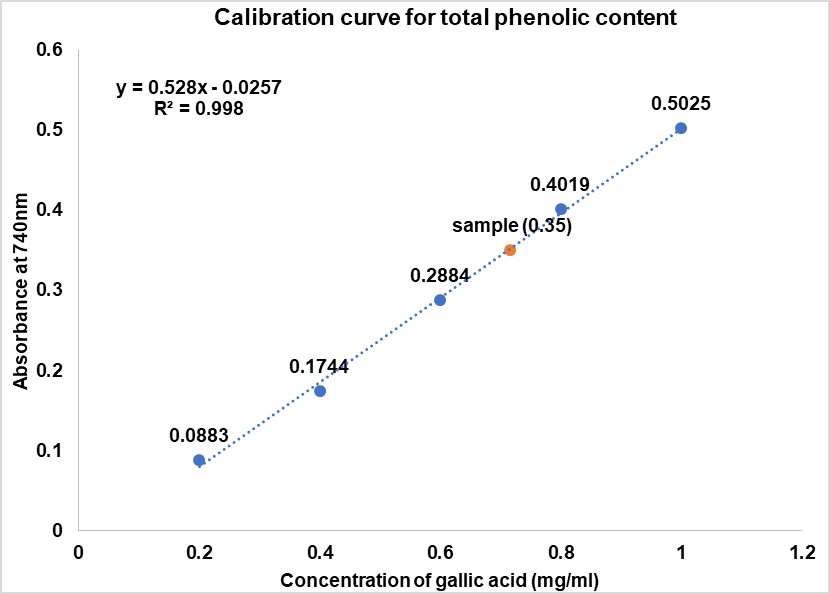
Fig. 1: Calibration curve for total phenolic content
Preliminary phytochemical screening of chamomile extract (table 1)
Flavonoids: The extract showed a distinct orange coloration upon addition of magnesium and concentrated HCl, indicating the presence of flavonoids. This color changes confirm the formation of flavonoid–metal complexes.
Tannins: Boiling the extract in distilled water, followed by the addition of 0.1% ferric chloride, results in the formation of blackish coloration. This reaction confirms the presence of tannins in the chamomile extract.
Glycosides: No significant color change is observed at the interface or in the upper layer upon the addition of glacial acetic acid, ferric chloride, and concentrated sulfuric acid. This suggests the absence of glycosides in the chamomile extract.
Coumarines: Under UV light, the extract treated with 1N sodium hydroxide exhibited a characteristic green fluorescence, confirming the presence of coumarins due to their UV-fluorescent lactone structure in alkaline medium.
Terpenoids: A reddish-brown coloration developed at the interface upon addition of concentrated sulfuric acid to the chloroform solution of the n-hexane extract, indicating the presence of terpenoids.
Saponins: Persistent foam formation is observed upon vigorous shaking of the aqueous extract, confirming the presence of saponins.
Table 1: Phytochemical analysis of chamomile flower extract
| S. No. | Phytochemical test | Result |
| 1 | Flavonoids | + |
| 2 | Tannins | + |
| 3 | Glycosides | – |
| 4 | Coumarins | + |
| 5 | Terpenoids | + |
| 6 | Saponins | + |
Evaluation of antioxidant activity of chamomile extract
The antioxidant potential of the hydro-alcoholic chamomile extract is assessed using the DPPH free radical scavenging assay. The absorbance of the control is recorded as 0.48, and the absorbance of the sample is 0.12 at 515 nm. The radical scavenging activity is calculated, that the result indicates that the chamomile extract exhibits significant antioxidant activity, with a DPPH scavenging effect of 75%, suggesting its potential for neutralizing free radicals.
HPLC analysis
High-Performance Liquid Chromatography (HPLC) analysis of the hydro-alcoholic extract of Matricaria chamomilla is performed to identify and compare the presence of selected flavonoids-Quercetin, Rutin, Naringin, Apigenin, and Morin. Identification is based on the comparison of retention times with those of standard compounds. The results indicate that the flavonoids are separated and identified under the chromatographic conditions used. Based solely on peak area comparison, Apigenin is found to be the most abundant flavonoid in the chamomile extract, followed by Morin, Naringin, Quercetin, and Rutin. This suggests that Apigenin may contribute significantly to the biological activity of the extract (table 2 and fig. 1-6).
Table 2: HPLC analysis of flavonoids in chamomile extract
| Compound | RT (Standard, min) | RT (Sample, min) | Area (Standard) | Area (Sample) |
| Quercetin | 18.88 | 18.23 | 606.184 | 27.888 |
| Rutin | 2.23 | 2.19 | 364.199 | 3.410 |
| Naringin | 14.45 | 14.95 | 50.376 | 11.339 |
| Apigenin | 20.01 | 20.07 | 1110.004 | 44.432 |
| Morin | 17.06 | 16.84 | 637.661 | 38.954 |
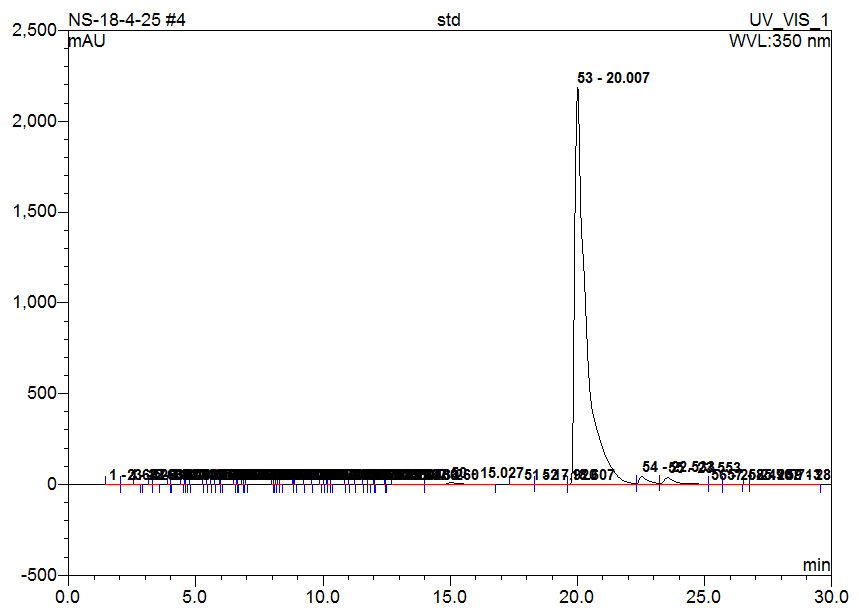
Fig. 1: Chromatogram of standard (Apigenin)
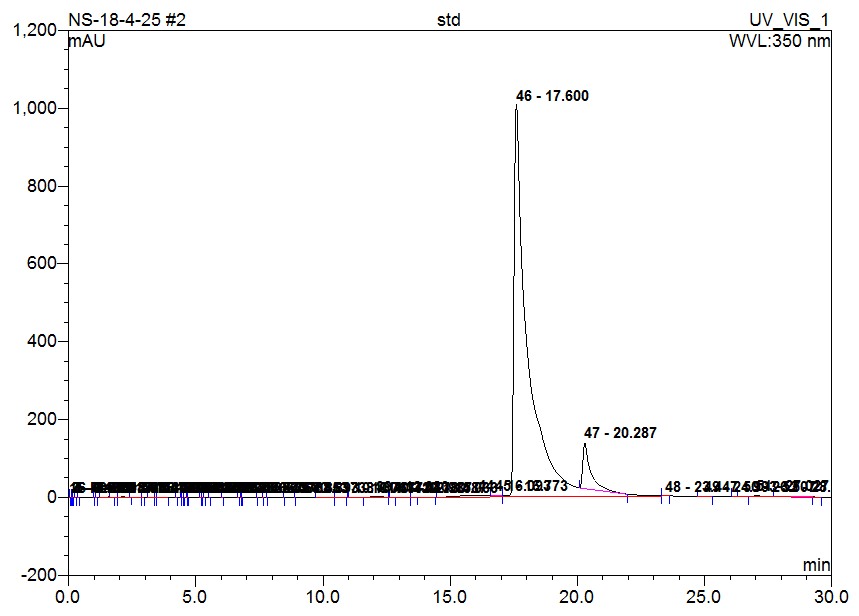
Fig. 2: Chromatogram of standard (Morin)
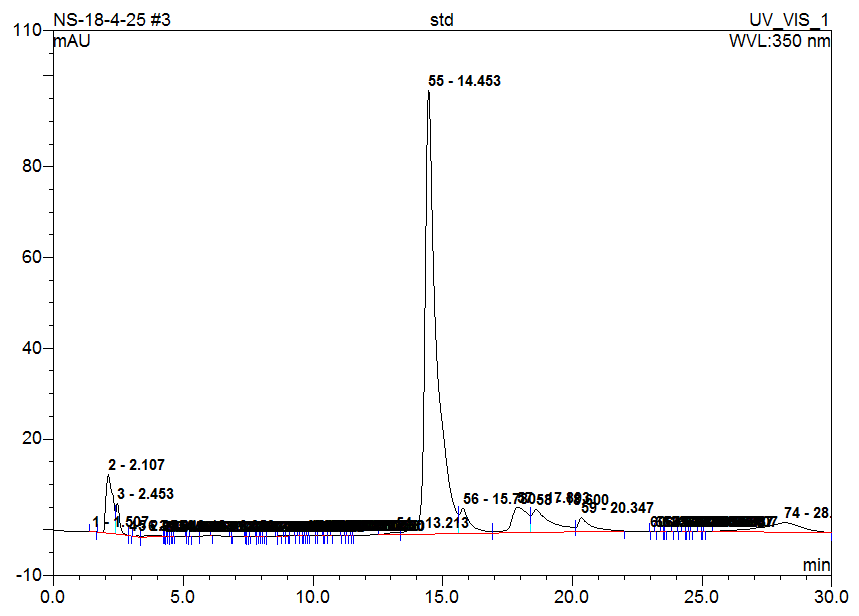
Fig. 3: Chromatogram of standard (Naringin)
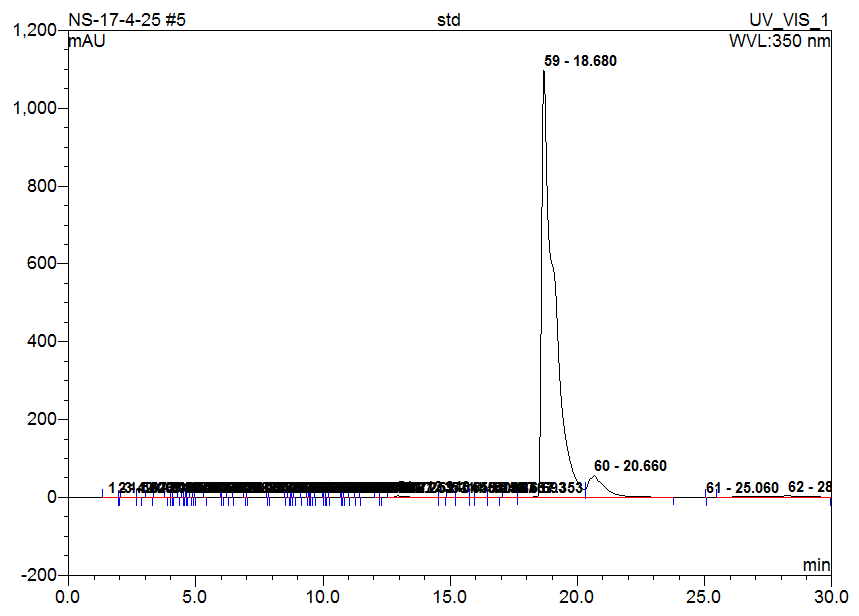
Fig. 4: Chromatogram of standard (Quercetin)
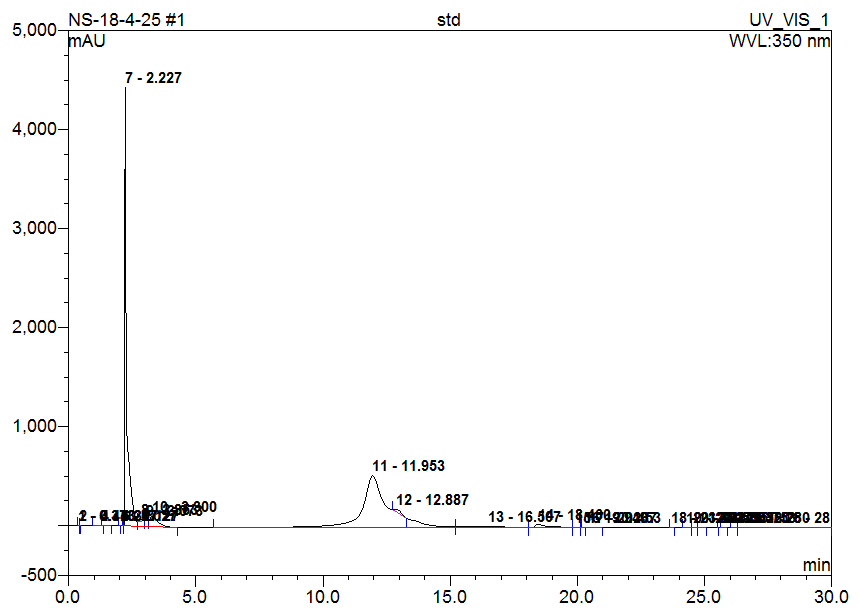
Fig. 5: Chromatogram of standard (Rutin)
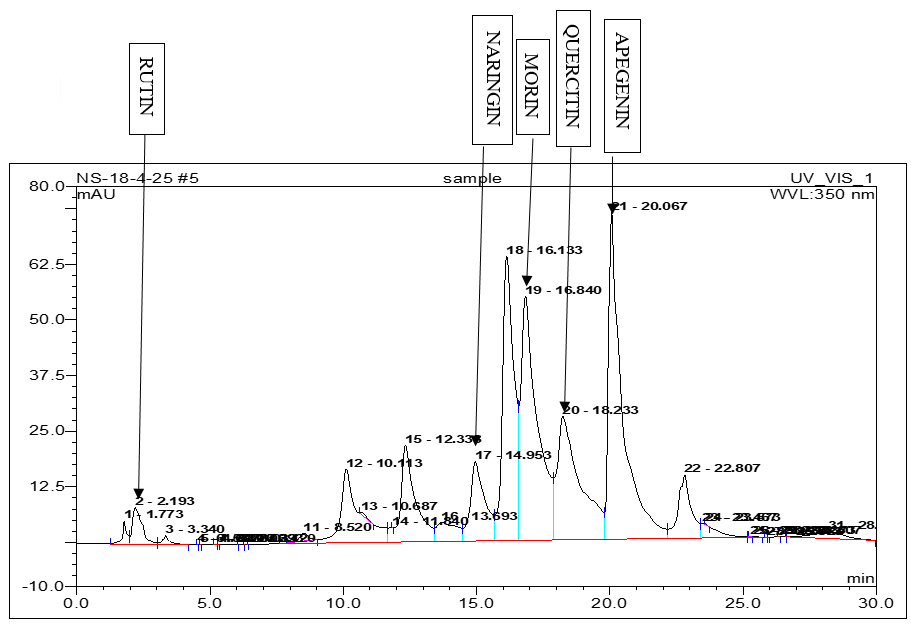
Fig. 6: Chromatogram of sample (Hydro-alcoholic extract)
GCMS analysis
The GC-MS analysis of the n-hexane extract of Matricaria chamomilla (chamomile) flowers revealed a diverse phytochemical profile, with approximately 65 compounds identified (table 3). These constituents encompass a variety of chemical groups, including alkanes (such as nonacosane, octane, and triacontane), terpenoids (including phytol, caryophyllene, and caryophyllene oxide), sterols and alcohols (notably stigmasterol and ergost-5-en-3-ol), fatty acid esters (such as hexadecanoic acid and its hexadecyl ester), and siloxanes (e. g., cyclooctasiloxane and hexadecamethyl-). Additionally, other hydrocarbons and derivatives like neophytadiene and squalene were also present in the extract.
Among these, several compounds are noteworthy for their documented anticancer properties. For instance, stigmasterol has been reported to exhibit cytotoxicity against breast cancer cell lines [12]. Phytol is recognized for its dual role in anticancer and anti-inflammatory pathways [13], while squalene serves as a chemopreventive agent and functions as an adjuvant in cancer therapy [14]. Caryophyllene oxide has demonstrated potent antilymphoma activity by inducing apoptosis and necrosis in cancer cells [15]. Furthermore, 13-Docosenamide (Z)-, also known as erucamide, exhibits both antimicrobial and anticancer activities [16]. These findings highlight the therapeutic potential of chamomile-derived compounds in the development of anticancer formulations.
Table 3: Identified compounds in GC-MS with their significance
| Compound name | Molecular formula | Structure | Type | Biological activity |
| Stigmasterol | C29H48O | 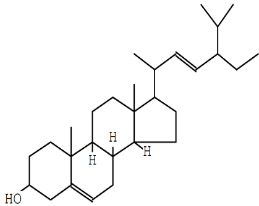 |
Sterol | Anticancer (cytotoxic to cancer cells) |
| Phytol | C20H40O | 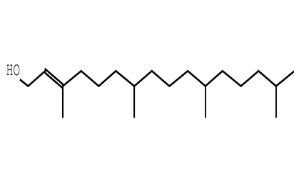 |
Diterpene alcohol | Anticancer, anti-inflammatory |
| Squalene | C30H50 | 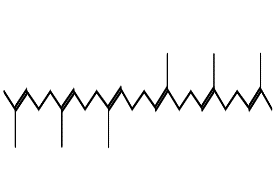 |
Triterpene | Chemopreventive, antioxidant |
| Caryophyllene oxide | C15H24O | 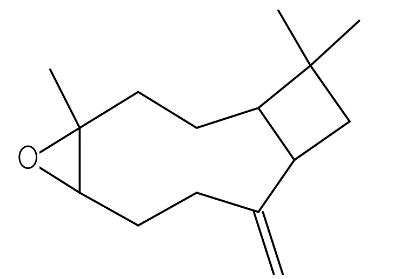 |
Sesquiterpene oxide | Antifungal, anticancer |
| 13-Docosenamide (Z)- | C22H43NO | 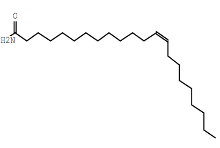 |
Fatty acid amide | Antimicrobial, anticancer |
| Neophytadiene | C20H38 | 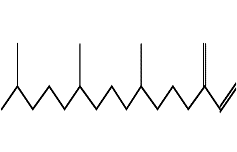 |
Hydrocarbon diterpene | Anti-inflammatory, antimicrobial |
| Isopropyl myristate | C17H34O2 | 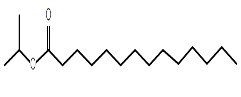 |
Ester | Enhances skin penetration, antimicrobial |
| Hexadecanoic acid, Hexadecyl ester | C32H64O2 | 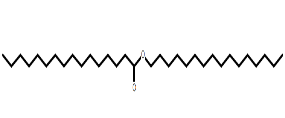 |
Fatty acid ester | Antioxidant, antimicrobial |
| Cyclooctasiloxane | C16H48O8Si8 | 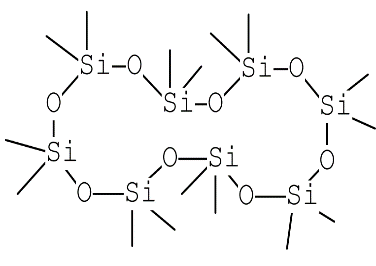 |
Siloxane | Antimicrobial |
| Linoleyl acetate | C20H36O2 | 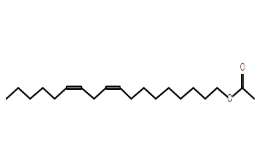 |
Fatty acid ester | Anti-inflammatory, antimicrobial |
| Germacrene D | C15H24 | 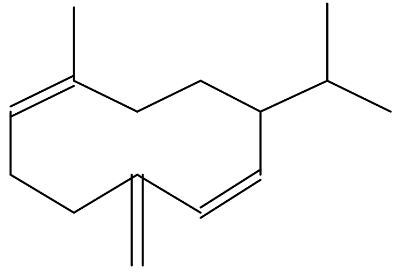 |
Sesquiterpene | Antimicrobial, anti-inflammatory |
| Ergost-5-en-3-ol | C28H48O | 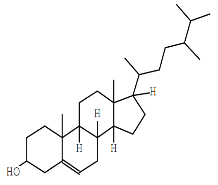 |
Sterol | Cytotoxic to cancer cells |
| Octanal | C8H16O | 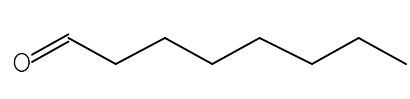 |
Aldehyde | Antimicrobial |
| 2-Heptanone | C7H14O | 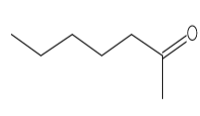 |
Ketone | Antimicrobial |
| Oxirane, hexadecyl- | C18H36O2 | 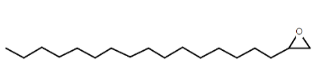 |
Epoxide | Antimicrobial |
DISCUSSION
The main aim of this study is to identify bioactive compounds present in Matricaria chamomilla (chamomile) flowers, which are widely known for their medicinal properties. Extraction is carried out in two phases—polar and non-polar. A hydro-alcoholic extract is prepared using ethanol and water in a 1:1 ratio, following the method described by Kumar et al. [8]. For non-polar compounds, n-hexane is used. After back extraction, the n-hexane layer turns pale yellow, indicating the presence of lipophilic compounds. To estimate the total phenolic content (TPC) of the hydro-alcoholic extract, Gallic acid is used as a standard. The TPC is found to be 782.1 mg GAE/g, which is relatively high and comparable to the 718 mg GAE/g reported by Park et al. [17] using fermented chamomile. This demonstrates that hydro-alcoholic extraction is effective for isolating phenolic compounds. Phytochemical analysis is conducted using the method of Natarajan et al. [10], originally applied to a different plant. The extract tests strongly positive for terpenoids and shows a mild presence of tannins, coumarins, and saponins. These findings are similar to those of Khan et al. [18]. Urolagin et al. [19] also report similar profiles, particularly in ethanol-based extracts. The antioxidant activity of the hydro-alcoholic extract is evaluated using the DPPH assay. At 10 µl** concentration, the extract shows 75.1% scavenging activity, reflecting strong antioxidant potential. This is higher than the 64.9% reported by Kumar et al. [8] and slightly lower than the 83.3% observed by Park et al. [17].
HPLC analysis identifies Apigenin as the predominant flavonoid, as indicated by the highest peak area (44.432) among the standards tested. This finding aligns with previous studies, including Kumar et al. [8], who reported apigenin-7-O-glucoside as the major compound (0.81% w/w) in hydro-alcoholic extracts, along with significant polyphenol content. Similarly, Qureshi et al. [9] and Kashchenko et al. [6] confirm apigenin as the most abundant flavonoid, followed by quercetin, luteolin, isorhamnetin, and kaempferol. GC-MS analysis of the n-hexane extract reveals a diverse phytochemical profile, with approximately 15 notable compounds identified. Stigmasterol is recognized for its cytotoxic effects against breast cancer cell lines [12]. Phytol plays a dual role in anticancer and anti-inflammatory pathways [13], while squalene acts as a chemopreventive agent and adjuvant in cancer therapy [14]. Caryophyllene oxide exhibits potent antilymphoma activity by inducing apoptosis and necrosis in cancer cells [15]. Additionally, 13-Docosenamide (Z)-, or erucamide, demonstrates anticancer activities [16]. These findings emphasize the therapeutic potential of chamomile-derived compounds, especially in the development of anticancer formulations.
CONCLUSION
The evaluation of chamomile flower extract using advanced analytical techniques provided valuable insights into its rich bioactive profile. Extraction methods effectively isolated key phytochemicals, including flavonoids, alkaloids, and terpenoids, known for their therapeutic effects. Total Phenolic Content (TPC) analysis and antioxidant assays confirmed strong antioxidant potential, indicating the extract’s role in combating oxidative stress. HPLC and GC-MS identified approximately 90 compounds, many with reported anticancer activity. These findings highlight chamomile’s chemical richness and support its potential for further research into cytotoxic, antimicrobial, hypoglycemic, and hypolipidemic properties.
ABBRIAVATIONS
Abbreviation-full form, HPLC-High-Performance Liquid Chromatography, GC-MS-Gas Chromatography–Mass Spectrometry, TPC-Total Phenolic Content, DPPH-2,2-Diphenyl-1-picrylhydrazyl, UV-Ultraviolet, EI-Electron Ionization, GAE-Gallic Acid Equivalent, RT-Retention Time, MC-Matricaria chamomilla L., CN-Chamaemelum nobile, TRACe-Toshvin Research and Application Centre, µl (or μl)-Microliter, ml-Milliliter, mg-Milligram, rpmRevolutions per minute, nm-Nanometer, °C-Degrees Celsius, min-Minute(s), Conc.-Concentrated, v/v-Volume/Volume (ratio), m/z-Mass-to-charge ratio, No.-Number, H2SO4-Sulfuric acid, NaOH Sodium hydroxide, UV-VisUltraviolet–Visible (Spectroscopy), NISTNational Institute of Standards and Technology (library).
ACKNOWLEDGEMENT
For the constant support and encouragement, all authors show their deepest gratitude to the Institute of Biosciences and Technology, MGM University, Chh. Sambhajinagar. Our sincere gratitude to the Toshvin Research and Application Centre (TRACe), Mumbai, for their support for the GC-MS analysis of this research work.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
Nidhi and Sanika collected the sample and prepared the crude extracts along with solvents. Vishal and Kushal performed the HPLC, Sanika and Nidhi, along with the scientist of Toshvin Research and Application Centre (TRACe), Mumbai, worked on GC-MS. Dr. Rajakumar supervised the overall process of the experiment and drafted the research paper. All authors contributed to the overall shaping of the research paper for publication.
CONFLICTS OF INTEREST
Declared none
REFERENCES
Dai YL, Li Y, Wang Q, Niu FJ, Li KW, Wang YY. Chamomile: a review of its traditional uses, chemical constituents, pharmacological activities and quality control studies. Molecules. 2022;28(1):133. doi: 10.3390/molecules28010133, PMID 36615326.
Helal MH, Badr SE, Abed Elaaty SA. Chemical characterization antioxidant anticancer and hypolipidemic activities of chamomile (Matricaria chamomillaL.). Nutr Res Food Sci J. 2021;4(2):1-8.
El Mihyaoui A, Esteves Da Silva JC, Charfi S, Candela Castillo ME, Lamarti A, Arnao MB. Chamomile (Matricaria Chamomilla L.): a review of ethnomedicinal use phytochemistry and pharmacological uses. Life (Basel). 2022;12(4):479. doi: 10.3390/life12040479, PMID 35454969.
Camara JS, Perestrelo R, Ferreira R, Berenguer CV, Pereira JA, Castilho PC. Plant-derived terpenoids: a plethora of bioactive compounds with several health functions and industrial applications a comprehensive overview. Molecules. 2024;29(16):3861. doi: 10.3390/molecules29163861, PMID 39202940.
Molnar M, Mendesevic N, Subaric D, Banjari I, Jokic S. Comparison of various techniques for the extraction of umbelliferone and herniarin in Matricaria chamomilla processing fractions. Chem Cent J. 2017;11(1):78. doi: 10.1186/s13065-017-0308-y, PMID 29086851.
Kaschenko NI, Olemnikov DN. Quantitative analysis of flavonoids in chamomile flowers (Matricaria Chamomilla L.) by microcolumn HPLC-UV. Russ J Bioorg Chem. 2017;43(7):783-9. doi: 10.1134/S106816201707007X.
Kumar VK, Kumar HC, Barangi VC, Aluri S, Reddy DK, Chandrashekara BM. Characterization of the phytochemicals in chamomile and evaluation of their biological activity. IOSR JPBS. 2023;18(4-1):26-33. doi: 10.9790/3008-1804012633.
Qureshi MN, Stecher G, Bonn GK. Determination of total polyphenolic compounds and flavonoids in Matricaria chamomella flowers. Pak J Pharm Sci. 2019;32(5):2163-5. PMID 31813883.
Natarajan M, Prasanthkumar V, Manju D, Umamaheswari S. Phytochemical analysis and antibacterial activity of different solanum species against Helicobacter pylori. Int J Pharm Biol Sci. 2018;8(1):335-40.
Rajurkar NS, Hande SM. Estimation of phytochemical content and antioxidant activity of some selected traditional Indian medicinal plants. Indian J Pharm Sci. 2011;73(2):146-51. doi: 10.4103/0250-474x.91574, PMID 22303056.
Bhattacharya S, Ghosh S. Stigmasterol shows cytotoxic activity against breast cancer cell lines. Biomed Pharmacother. 2014;68(3):407-15.
De Morais SM, Magalhaes DV, Moreira MR, Vieira IG, Almeida RR, Guimaraes AC. Antinociceptive and anti-inflammatory effects of phytol. Braz J Pharmacogn. 2014;24(2):214-20.
Reddy LH, Couvreur P. Squalene: a natural triterpene for use in disease management and therapy. Adv Drug Deliv Rev. 2009;61(15):1412-26. doi: 10.1016/j.addr.2009.09.005, PMID 19804806.
Gertsch J, Leonti M, Raduner S, Racz I, Chen JZ, Xie XQ. Beta-caryophyllene is a dietary cannabinoid. Proc Natl Acad Sci USA. 2008;105(26):9099-104. doi: 10.1073/pnas.0803601105, PMID 18574142.
Ghosh S, Bhattacharyya S. Antimicrobial and anticancer properties of erucamide. J Appl Pharm Sci. 2020;10(4):101-6.
Park EH, Bae WY, Eom SJ, Kim KT, Paik HD. Improved antioxidative and cytotoxic activities of chamomile (Matricaria chamomilla) florets fermented by Lactobacillus plantarum KCCM 11613P. J Zhejiang Univ Sci B. 2017;18(2):152-60. doi: 10.1631/jzus.B1600063, PMID 28124843.
Khan N, Kalam MA, Alam MT, Haq SA, Showket W, Dar ZA. Drug standardization through pharmacognostic approaches and estimation of anticancer potential of chamomile (Matricaria Chamomilla L.) using prostate-cancer cell lines: an in vitro study. J Cancer. 2023;14(3):490-504. doi: 10.7150/jca.77110, PMID 36860921.
Urolagin D, Raza AA, Panda S, HL D, Boro M. Pharmacognostic and pharmacological review of Matricaria chamomilla. Int J Creat Res Thoughts. 2024;12(4):447-51.
Vadivelan Ramachandran, Gautam Adhikari. Hydroalcoholic extract of Matricaria chamomilla linn. Ameliorates lipids lipoproteins and paraoxonase in isoproterenol induced myocardial infarction in Wistar rats. Asian J Pharm Clin Res. 2019;12(4):125-9. doi: 10.22159/ajpcr.2019.v12i4.31145.
Satya Prasad B, Jayakumari S. Quantification of anticoagulants dabigatran Rivaroxaban and prasugrel by chromatographic and spectrometric techniques review. Asian J Pharm Clin Res. 2019;12(4):1-8. doi: 10.22159/ajpcr.2019.v12i4.29954.