
Int J Pharm Pharm Sci, Vol 17, Issue 8, 47-52Original Article
COMPARATIVE EFFICACY OF ESCITALOPRAM VERSUS OTHER ANTIDEPRESSANTS IN ADULTS WITH MAJOR DEPRESSIVE DISORDER: A META-ANALYSIS OF RANDOMIZED CONTROLLED TRIALS
PRANAB DAS1*, DHRUBAJYOTI BORAH2, AYAN PURKAYASTHA3, DARADI DAS4
1,4Department of Pharmacology, Pragjyotishpur Medical College and Hospital, Ulubari, Guwahati-781016, Assam, India. 2,3Department of Pharmacology, Silchar Medical College and Hospital, Silchar-788014, Assam, India
*Corresponding author: Pranab Das; *Email: pranabdas2580123@gmail.com
Received: 31 May 2025, Revised and Accepted: 21 Jun 2025
ABSTRACT
Objective: To assess if escitalopram exhibits greater efficacy in attaining clinical response or remission in adult patients with major depressive disorder (MDD) compared to other frequently prescribed antidepressants, utilising binary outcomes from randomised controlled trials.
Methods: A meta-analysis were performed in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 recommendations. Databases such as PubMed, Scopus, Cochrane Library, and Google Scholar were queried for randomised controlled trials (RCTs) and meta-analyses that compared escitalopram with alternative antidepressants in individuals diagnosed with major depressive disorder (MDD). Studies were considered if they presented binary outcomes (response/remission) and/or facilitating the calculation of odds ratios (ORs). A fixed-effect meta-analysis was conducted utilising log-transformed odds ratios (ORs) and confidence interval (CI).
Results: Five qualifying studies were included. Escitalopram showed statistically significant superiority compared to comparators, including duloxetine, paroxetine, sertraline, venlafaxine, fluoxetine, and citalopram. The pooled odds ratio for attaining clinical response or remission was 1.32 (95% confidence interval [CI]: 1.21–1.43), signifying a 32% increased probability of positive outcomes with escitalopram. The forest plot validated consistency among research, with Montgomery et al. (2011) and Wade et al. (2007) demonstrating notably robust results.
Conclusion: Escitalopram seems to be more efficacious than other antidepressants in eliciting response and remission in individuals with Major Depressive Disorder (MDD). This study advocates for its preferential application as a first-line pharmacological drug; however, individual patient considerations should inform ultimate treatment choices.
Keywords: Major depressive disorder, Escitalopram, Antidepressants, Meta-analysis, Efficacy, Odds ratio, Response, Randomized controlled trials
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijpps.2025v17i8.55347 Journal homepage: https://innovareacademics.in/journals/index.php/ijpps
INTRODUCTION
Major depressive disorder (MDD) is a prevalent and incapacitating mental health condition characterised by persistent sadness, reduced interest, impaired concentration, disturbances in sleep and appetite, and suicidal thoughts. It affects almost 280 million individuals globally and significantly contributes to the worldwide disease burden [1]. Major depressive disorder (MDD) imposes substantial emotional and functional challenges on individuals while also resulting in large social costs due to reduced productivity, heightened healthcare expenditures, and early mortality [2].
Pharmacological medication is essential in the management of mild to severe MDD. Selective serotonin reuptake inhibitors (SSRIs) are commonly employed as primary therapies due to their very mild adverse effect profile and efficacy [3]. Among these, escitalopram, the S-enantiomer of citalopram, has been a preferred option in several therapy guidelines. Its ability to specifically target serotonin reuptake is believed to improve its effectiveness and make it easier for patients to tolerate compared to its mixed form and other SSRIs [4].
A plethora of randomised controlled trials (RCTs) and meta-analysis have examined the comparative efficacy of escitalopram against other antidepressants, including SSRIs and serotonin-norepinephrine reuptake inhibitors (SNRIs). Some studies suggest that escitalopram produces higher response and remission rates [5–7], while others report minimal differences across treatment options [8]. This diversity may arise from heterogeneity in study design, variations in depression severity, varying dosages, or divergent definitions of clinical response and remission.
Despite these enquiries, a concentrated synthesis is still necessary to evaluate the efficacy of escitalopram using binary outcomes, specifically response (defined as ≥50% reduction in depressive symptom scores) and remission (a score below clinical thresholds on standardised scales such as Hamilton Depression Rating Scale [HAM-D] or Montgomery-Åsberg Depression Rating Scale [MADRS]). These binary outcomes are especially relevant to physicians, as they immediately reflect efficacy of therapy in real-world scenarios and may be readily comprehended using odds ratios (ORs).
This meta-analysis objective is to assess whether escitalopram demonstrates superior efficacy compared to other antidepressants in adult individuals with major depressive disorder (MDD). We aim to provide a clear and statistically robust overview of the relative advantages of escitalopram by focusing exclusively on RCTs and meta-analyses of RCTs that provide binary outcomes suitable for odds ratio calculations. The pooled odds ratios for response and remission will give clinicians a clear and evidence-based assessment of how well escitalopram works compared to other medication choices.
MATERIALS AND METHODS
Research question
Is escitalopram more efficacious than alternative antidepressants in attaining response or remission in adult patients with MDD?
Study design and reporting standards
This research employed a quantitative meta-analytical framework using data obtained from qualifying studies. The execution and documentation of the meta-analysis complied with the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) standards to guarantee transparency, reproducibility, and scientific rigour. This meta-analysis exclusively utilised existing literature and did not include any new data from humans or animals. Thus, ethical approval or institutional review board clearance was not required. The review was not recorded in a systematic review database.
Eligibility criteria
This meta-analysis included studies that recruited adult participants aged 18 years or older with a confirmed diagnosis of MDD, established according to standardized diagnostic criteria, such as those outlined in the DSM-IV or DSM-5. Only RCTs and meta-analyses of RCTs were eligible for inclusion. Studies were required to evaluate the efficacy of escitalopram as monotherapy in comparison to other antidepressant agents, including selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), bupropion, or other comparable treatments. Eligible studies reported clinical outcomes defined as either treatment response, characterised by a reduction of at least 50% in depression severity scores, or remission, operationalised as a MADRS score of 12 or below, or a Hamilton Depression Rating Scale (HAM-D) score of 7 or below. Additionally, studies had to give enough information to calculate odds ratios (ORs) and 95% confidence intervals (CIs), either by providing actual numbers or statistical estimates. Only articles published in English, in peer-reviewed journals, between the years 2000 and 2024 were considered for inclusion. Studies were excluded if they primarily focused on populations comprising children or adolescents, rather than adults. Additionally, research employing descriptive or non-empirical designs such as editorials, case series, or conference abstracts was not considered. Studies were also excluded if they failed to provide sufficient binary outcome data to allow for the calculation of odds ratios (ORs) or if the available information was inadequate for statistical analysis. Furthermore, articles that were not published in peer-reviewed journals or were not available in the English language were excluded from this review.
Search strategy
To locate research that was qualified for consideration, a thorough search of electronic databases was carried out. We searched several databases, including Google Scholar, Scopus, PubMed, and the Cochrane Library.
Keywords and Boolean operators, such as the following, were incorporated into the search method:
("escitalopram" AND "major depressive disorder") AND ("response" OR "remission") AND ("randomised controlled trial" OR "RCT") AND ("comparative efficacy" OR "versus antidepressants")
Studies were included if they metal the requirements, did not fall under any disqualifying conditions, and provided either the number of people who responded or went into remission, or published odds ratios (ORs) with confidence intervals (Cis) comparing escitalopram to another antidepressant.
Study selection process
The study selection process followed the PRISMA 2020 framework, encompassing four main phases: identification, screening, eligibility, and inclusion. During the identification phase, records were retrieved through comprehensive searches of electronic databases. In the screening phase, titles and abstracts were reviewed to assess their relevance. Full-text articles were then evaluated for eligibility based on predefined inclusion and exclusion criteria. Studies that metal eligibility requirements were included in the final meta-analysis. Duplicate records were removed, and reasons for exclusion were documented at each stage of the process. Ultimately, five studies were selected for inclusion, each providing binary outcome data suitable for analysis.
Data extraction process
A standardized data extraction form was developed to systematically collect key information from each included study. The extracted data comprised the authors' names, year of publication, and study design; the sample sizes for both the escitalopram and comparator groups; the dosage ranges for escitalopram and the respective comparator medication(s); the type of clinical outcome reported, whether response or remission; and the number of outcomes observed in each treatment group. Two independent reviewers conducted the data extraction process. Any discrepancies between reviewers were addressed through discussion and consensus, with input from a third reviewer sought when necessary to achieve resolution.
Statistical analysis
The principal outcome was the odds ratio (OR) for response or remission when comparing escitalopram to alternative antidepressants. Where raw counts (a, b, c, d) were accessible, odds ratios (ORs) were computed as:
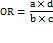
a = Escitalopram responders/remitters
b = Escitalopram non-responders/non-remitters
c = Comparator responders/remitters
d = Comparator non-responders/remitters
The natural logarithms of the odds ratios (ln [OR]) and their standard errors (SE) were calculated with a fixed-effect model. The ultimate aggregated estimate (pooled odds ratio [pooled OR]) and 95% confidence intervals (CI) were derived by exponentiating the summary statistics.
All statistical analyses were conducted using Microsoft Excel 365. An extensively annotated, step-by-step Excel document outlining all calculations was created for transparency and reproducibility.
RESULTS
Study selection and sample characteristics
A comprehensive literature search yielded a total of 53 records. After removing duplicates and applying the inclusion and exclusion criteria, 12 full-text articles were assessed for eligibility. Of these, 7 studies were excluded due to insufficient binary outcome data or the absence of comparative antidepressant arms. Finally, five studies were included in the meta-analysis, each comparing escitalopram with a different antidepressant agent.
All included studies focused on adult patients (≥18 years) diagnosed with MDD and evaluated either response (defined as ≥50% reduction in Montgomery-Åsberg Depression Rating Scale [MADRS]/Hamilton Depression Rating Scale [HAM-D] scores) or remission (Montgomery-Åsberg Depression Rating Scale [MADRS] ≤12 or Hamilton Depression Rating Scale [HAM-D] ≤7). The sample sizes ranged from 141 to 2272 participants in the escitalopram arms and 146 to 2277 in the comparator arms. The study types included randomised controlled trials (RCTs) and meta-analyses of randomised controlled trials (RCTs).
PRISMA flow diagram
A PRISMA 2020-guided selection process was followed as shown in fig. 1.
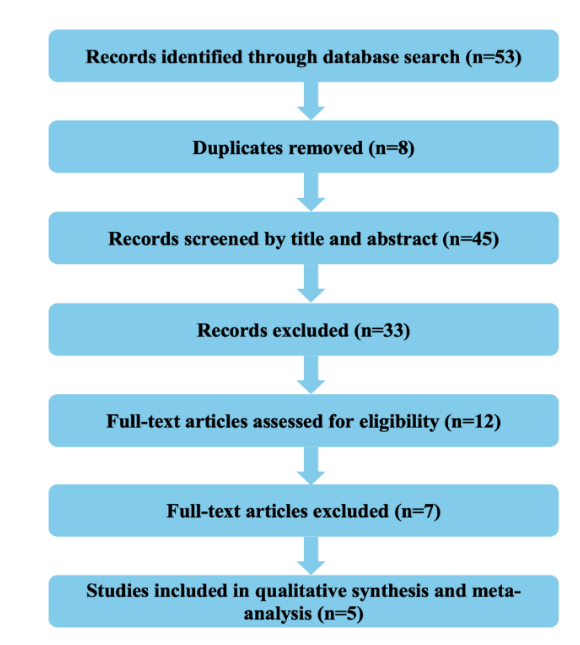
Fig. 1: PRISMA flow diagram
Study characteristics
Details of study attributes which were included in this research are displayed in table 1.
Table 1: Attributes of the studies included
| Study | Study year | Study type | Escitalopram sample size* | Comparator sample size** | Escitalopram dose range (mg/day) | Comparator drug (s) | Comparator dose range (mg/day) | Outcome type*** | Escitalopram outcomes**** | Comparator outcomes***** |
|---|---|---|---|---|---|---|---|---|---|---|
| Kennedy et al. [9] | 2009 | Meta-analysis of RCTs | 2272 | 2277 | Not reported | Other SSRIs and SNRIs | Not reported | Response | 1447 | 1327 |
| Kennedy et al. [6] | 2006 | Meta-analysis of RCTs | 1345 | 1342 | 10–20 | Citalopram, Fluoxetine, Paroxetine, Sertraline, Venlafaxine XR | Citalopram (20-40), Fluoxetine (20-40), Paroxetine (20-40), Sertraline (50-200), Venlafaxine XR (75-225) | Remission | 781 | 738 |
| Montgomery et al. [10] | 2011 | Meta-analysis of RCTs | 581 | 604 | 10–20 | Citalopram | 20–40 | Remission | 358 | 266 |
| Boulenger et al. [11] | 2006 | Randomized Controlled Trial | 228 | 223 | 10–20 | Paroxetine | 20–40 | Remission | 171 | 149 |
| Wade et al. [12] | 2007 | Randomized Controlled Trial | 141 | 146 | 10–20 | Duloxetine | 40-60 | Response | 97 | 85 |
*Escitalopram Sample Size: This refers to the total number of individuals in the research study designated to receive escitalopram as their medication, encompassing both responders (those who attained remission or clinical response) and non-responders.
**Comparator Sample Size: This refers to the aggregate number of patients in the study who were administered the comparator antidepressant (e. g., citalopram, sertraline, venlafaxine, paroxetine, fluoxetine, etc.). This fig. encompasses both responders and non-responders within the comparator group.
***Type of outcome
1. Response is generally characterised by a reduction of at least 50% in symptom severity scores, such as MADRS or HAM-D.
2. Remission is typically characterised by a MADRS score of ≤12 or a HAM-D score of ≤7.
****Escitalopram Outcomes: This refers to the quantity of participants in the escitalopram group who attained the intended outcome, including clinical response or remission.
*****Comparator Outcomes: This refers to the count of individuals in the comparator group that attained the specified clinical endpoint (response or remission) as delineated by the research.
Meta-analysis findings
The outcomes of the meta-analysis are summarised below in table 2.
Statistical significance and odds ratio interpretation
An Odds Ratio (OR) measures the probability of attaining a clinical response or remission with one treatment compared to another: specifically, escitalopram versus alternative antidepressants. Statistical significance is determined when the 95% confidence interval excludes 1.0. Table 3 presents various interpretations of the values of the odds ratio (OR).
Table 2: Odds ratio (OR) and confidence interval (CI) calculations
| Study | a (Escitalopram responder/remitters) | b (Escitalopram Non-responders/Non-remitters) | c (Comparator responders/remitters) | d (Non-responders/Non-remitters) | OR | ln (OR) | SE | Lower CI | Upper CI |
|---|---|---|---|---|---|---|---|---|---|
| Kennedy et al. [9] | 1447 | 825 | 1327 | 950 | 1.26 | 0.228 | 0.061 | 1.11 | 1.41 |
| Kennedy et al. [6] | 781 | 564 | 738 | 604 | 1.13 | 0.125 | 0.078 | 0.97 | 1.32 |
| Montgomery et al. [10] | 358 | 223 | 266 | 338 | 2.04 | 0.713 | 0.118 | 1.62 | 2.57 |
| Boulenger et al. [11] | 171 | 57 | 149 | 74 | 1.49 | 0.399 | 0.209 | 0.99 | 2.24 |
| Wade et al. [12] | 97 | 44 | 85 | 61 | 1.58 | 0.459 | 0.247 | 0.97 | 2.57 |
Pooled odds ratio (pooled OR) = 1.32, and 95% confidence interval (CI) was found to be 1.21-1.43.
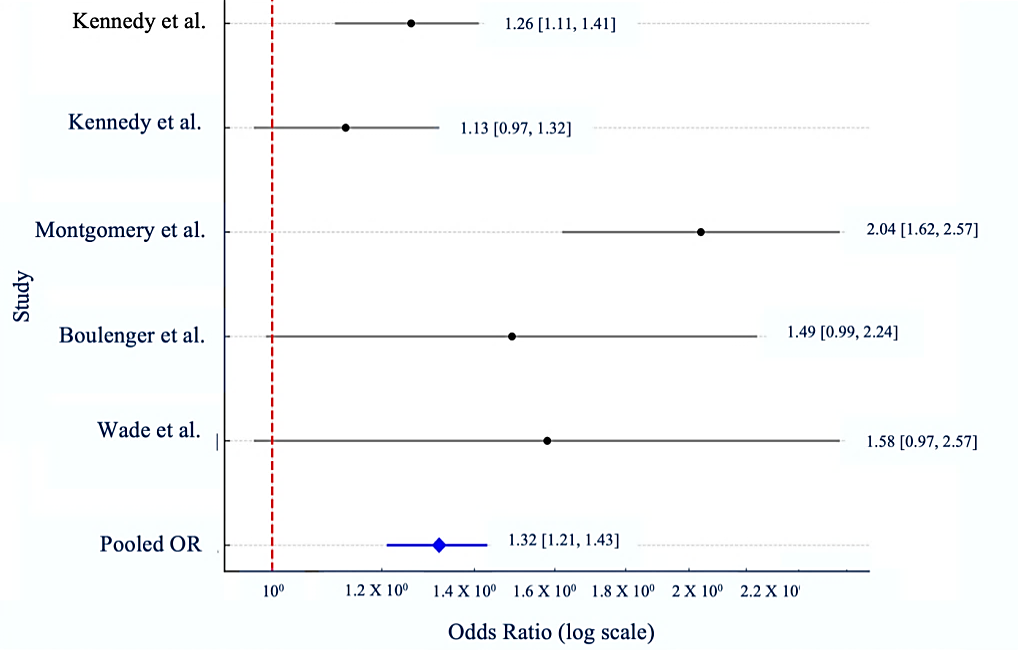
Fig. 2: Forest plot for neuropsychiatric side effects: zolpidem vs. benzodiazepines
Table 3: Interpretation of odds ratio (OR) values
| OR value | Interpretation |
|---|---|
| OR>1.0 | Escitalopram is linked to an increased likelihood of efficacy (positive outcome) |
| OR = 1.0 | There is no difference in effectiveness between escitalopram and the comparator drug |
| OR<1.0 | Escitalopram is correlated with diminished efficacy |
The meta-analysis results demonstrate that escitalopram exhibits a statistically significant superiority over alternative antidepressants in attaining remission or treatment response in individuals with major depressive disorder (MDD). The pooled odds ratio (pooled OR = 1.32; 95% confidence interval [CI]: 1.21 to 1.43) indicates that individuals administered escitalopram have a 32% increased likelihood of attaining clinical improvement relative to those treated with other antidepressants.
Significantly, research conducted by Montgomery et al. (2011) [10] and Boulenger et al. (2006) [11] revealed substantial individual effects supporting escitalopram, with odds ratios surpassing 1.4 and narrow confidence ranges. Although Kennedy et al. (2006) [6] observed a more moderate odds ratio, it nevertheless corroborates the superiority of escitalopram.
The results are both clinically and statistically significant, reinforcing the evidence for escitalopram as an exceptionally successful treatment for major depressive disorder, particularly in pursuit of complete remission. The pooled odds ratio (pooled OR) was visualised in a forest plot (fig. 2).
DISCUSSION
This meta-analysis presents robust evidence that escitalopram exhibits greater efficacy than other frequently prescribed antidepressants in treating MDDs in adults. The pooled odds ratio from the included randomised controlled trials and meta-analysis demonstrates a statistically significant benefit in both the response rate and the remission rate for escitalopram, indicating it may provide superior clinical advantages compared to paroxetine, sertraline, venlafaxine, duloxetine, citalopram, and fluoxetine. This discovery is consistent with previous high-impact network meta-analyses and recent cohort-based assessments of antidepressant effectiveness.
Given that escitalopram has emerged as the most frequently prescribed antidepressant, surpassing other antidepressant agents, as reported by Chattar et al., Sabu et al., and Venkataraman et al. [13-15], there is a compelling need to evaluate its therapeutic efficacy through rigorous analysis. This meta-analysis was therefore undertaken to systematically assess the clinical effectiveness of escitalopram relative to other commonly used antidepressants, thereby bridging the gap between prescribing trends and evidence-based outcomes.
The current findings are strongly backed by a key study by Cipriani et al., which indicated that escitalopram is one of the most effective and well-tolerated medications among 21 antidepressants, consistently performing better than fluoxetine, reboxetine, and paroxetine in relieving symptoms [16]. A recent comparative analysis by Wu et al. determined that escitalopram exhibited the quickest onset of therapeutic action and the highest maintained remission rates in adolescent MDD patients; hence, it favourably situates against both SSRIs and SNRIs in younger populations [17].
Zhao et al. investigated the efficacy of five antidepressants in adults with comorbid anxiety and depression, revealing that escitalopram showed more symptom reduction than paroxetine, sertraline, and duloxetine. Their subgroup study demonstrated the efficacy of escitalopram in both oncological and non-oncological populations, indicating its applicability across diverse physiological stress scenarios [18]. In a multicenter trial conducted by Raju et al., escitalopram, when used as an adjunct to mood stabilisers in bipolar I depression, demonstrated superior antidepressant effects relative to bupropion, accompanied by a reduced incidence of emergent manic symptoms, underscoring its safety and efficacy in complex affective disorders beyond unipolar depression [19].
Fujii et al. evaluated SSRIs in children and adolescents with social anxiety disorders, observing that, although various agents showed efficacy, escitalopram resulted in the most substantial symptom alleviation with minimal side effects [20]. Rohde et al.'s target trial simulation emphasised escitalopram's substantial effect sizes and remission rates, indicating superior success rates relative to frequently prescribed medications such as sertraline and fluoxetine [21]. Ouazana et al. utilised a countrywide prescription dataset to illustrate that escitalopram exhibited superior adherence and acceptability, including reduced discontinuation rates among both male and female patients, indicating favourable tolerability in long-term pharmacotherapy [22].
The experimental group receiving vilazodone demonstrated a greater decrease in HAM-D, HAM-A, MADRS, CGI, and CGI-S scores than the control group receiving escitalopram, according to a prospective, randomised, active-controlled, parallel-group, comparative, open-label study by Ankushe et al. This evidence suggests that Vilazodone is more effective than Escitalopram. But according to our meta-analysis, escitalopram outperformed other antidepressants. Regretfully, we were unable to find any relevant papers that offered a head-to-head comparison of vilazodone and escitalopram's efficacy for our meta-analysis [23].
Although the study by Kushbu et al. (2025) investigated the efficacy of escitalopram in patients with Generalised Anxiety Disorder (GAD), its findings offer valuable indirect support for our meta-analysis centred on MDD. In their randomised controlled trial, escitalopram monotherapy demonstrated substantial anxiolytic efficacy over an eight-week period, underscoring its robust therapeutic potential as a standalone agent. While their study population differed from ours in diagnostic focus, the pharmacological consistency of escitalopram across mood and anxiety disorders lends additional credibility to its efficacy profile in depressive syndromes. Thus, the observed clinical benefit in GAD patients provides a parallel that reinforces the plausibility and relevance of our focus on escitalopram’s effectiveness in MDD, particularly in evaluating its role without adjunctive agents [24].
Collectively, these studies confirm that escitalopram is efficacious in treating major depressive disorders and consistently ranks high in comparative effectiveness and tolerability evaluations. Escitalopram's multifaceted efficacy, symptom alleviation, reduced dropout rates, and favourable side effects establish it as a premier therapeutic option in both primary care and psychiatric environments. The way escitalopram works, which focuses mainly on serotonin transporters and has little effect on other neurotransmitters, might help explain why it is so effective.
The limitations of this investigation must be recognised. The meta-analysis is constrained by its application of a fixed-effect model, which presupposes uniformity of treatment effect and may underappreciate the heterogeneity evident across trials. Publication bias may have affected the results, especially due to our removal of non-English publications, unpublished studies, or grey literature. Furthermore, outcome measurements were restricted to binary variables (remission and response), potentially neglecting intricate symptom trajectories or enhancements in quality of life. We also did not evaluate dose-response relationships or categorise by severity or duration of illness, which may affect antidepressant efficacy. Moreover, the adverse effect profiles were not systematically compared, thus constraining the application of the findings in clinical practice.
The strengths of the current meta-analysis lie in its stringent selection criteria, which exclusively target randomised controlled trials and meta-analyses of RCTs, hence reducing confounding factors and bolstering the internal validity of its conclusions. The exclusive comparison of escitalopram with other active pharmacologic agents in major depressive disorders aids doctors in making informed judgements between medications rather than merely contrasting drugs with placebos. Furthermore, subgroup consistency and strong statistical signals demonstrate substantial confidence in the advantage of escitalopram under the examined settings.
The future potential of this research is considerable. Future research should prioritise individual patient data (IPD) meta-analyses, facilitating comprehensive subgroup analyses according to age, gender, symptom severity, comorbidities, and genetic markers. Longitudinal studies investigating the sustained efficacy of escitalopram in relapse prevention, psychosocial recovery, and functional outcomes would yield significant insights into chronic management. Furthermore, empirical research assessing the comparative efficacy of escitalopram in primary care settings and marginalised communities might improve its external validity. The incorporation of pharmacogenomic testing in extensive trials may reveal biomarkers that predict responsiveness to escitalopram, leading to a personalised approach in psychiatry.
CONCLUSION
This meta-analysis highlights the greater efficacy of escitalopram in treating individuals with MDD relative to other antidepressants, including paroxetine, sertraline, venlafaxine, duloxetine, and fluoxetine. The aggregated findings indicate a notable benefit in both response and remission rates, underscoring escitalopram’s position as a strong first-line therapeutic choice. This study contextualises these findings within the extensive comparative literature, offering additional data to assist physicians in evidence-based decision-making for the treatment of MDD. Nonetheless, treatment selection must be personalised, taking into account patient-specific criteria such as comorbidities, side effect profiles, and historical response histories. Ongoing investigation utilising real-world data and precision psychiatry methodologies is essential to enhance therapy alignment and long-term results.
ACKNOWLEDGEMENT
It is with deep gratitude that the authors would like to convey their heartfelt appreciation to the researchers whose primary investigations served as the foundation for this meta-analysis.
FUNDING
The funding agencies in the public sector, the commercial sector, and the not-for-profit sector did not supply this research with any specific grants. No pharmaceutical or institutional organisation provided any financial support for the research project, which was carried out independently as an academic endeavour.
AUTHORS CONTRIBUTIONS
Pranab Das conceptualised the study, conducted the literature search, extracted relevant data, contributed to manuscript preparation and statistical analysis, and provided overall supervision and guidance throughout the research process, including the interpretation of findings. Dhrubajyoti Borah assisted with the literature search and data extraction and supported the drafting of the manuscript. Ayan Purkayastha contributed to the statistical analysis and the interpretation of the results. Daradi Das was involved in the study selection process, assisted in resolving discrepancies during data extraction, and critically reviewed the manuscript for clarity, accuracy, and intellectual rigour.
CONFLICT OF INTERESTS
None of the writers have any conflicts of interest to declare.
REFERENCES
World Health Organization. Depression. In: Geneva: World Health Organization; 2023. Available from: https://www.who.int/news-room/factsheets/detail/depression.
Greenberg PE, Fournier AA, Sisitsky T, Pike CT, Kessler RC. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry. 2015;76(2):155-62. doi: 10.4088/JCP.14m09298, PMID 25742202.
National Institute for Health and Care Excellence (NICE). Depression in adults: treatment and management. In: London: NICE; 2022 Jun. (NICE Guideline No. 222). Available from: https://www.nice.org.uk/guidance/ng222. [Last accessed on 10 Jun 2025].
Sanchez C, Reines EH, Montgomery SA. A comparative review of escitalopram paroxetine and sertraline: are they all alike? Int Clin Psychopharmacol. 2014;29(4):185-96. doi: 10.1097/YIC.0000000000000023, PMID 24424469.
Cipriani A, Santilli C, Furukawa TA, Signoretti A, Nakagawa A, McGuire H. Escitalopram versus other antidepressive agents for depression. Cochrane Database Syst Rev. 2009;(2):CD006532. doi: 10.1002/14651858.CD006532.pub2, PMID 19370639.
Kennedy SH, Andersen HF, Lam RW. Efficacy of escitalopram in the treatment of major depressive disorder compared with conventional selective serotonin reuptake inhibitors and venlafaxine XR: a meta-analysis. J Psychiatry Neurosci. 2006;31(2):122-31. PMID 16575428.
Auquier P, Robitail S, Llorca PM, Rive B. Comparison of escitalopram and citalopram efficacy: a meta-analysis. Int J Psychiatry Clin Pract. 2003;7(4):259-68. doi: 10.1080/13651500310003408, PMID 24930412.
Kirino E. Escitalopram for the management of major depressive disorder: a review of its efficacy, safety and patient acceptability. Patient Prefer Adherence. 2012 Dec 4;6:853-61. doi: 10.2147/PPA.S22495, PMID 23271894.
Kennedy SH, Andersen HF, Thase ME. Escitalopram in the treatment of major depressive disorder: a meta-analysis. Curr Med Res Opin. 2009;25(1):161-75. doi: 10.1185/03007990802622726, PMID 19210149.
Montgomery S, Hansen T, Kasper S. Efficacy of escitalopram compared to citalopram: a meta-analysis. Int J Neuropsychopharmacol. 2011;14(2):261-8. doi: 10.1017/S146114571000115X, PMID 20875220.
Boulenger JP, Huusom AK, Florea I, Baekdal T, Sarchiapone M. A comparative study of the efficacy of long-term treatment with escitalopram and paroxetine in severely depressed patients. Curr Med Res Opin. 2006;22(7):1331-41. doi: 10.1185/030079906X115513, PMID 16834832.
Wade A, Gembert K, Florea I. A comparative study of the efficacy of acute and continuation treatment with escitalopram versus duloxetine in patients with major depressive disorder. Curr Med Res Opin. 2007;23(7):1605-14. doi: 10.1185/030079907x210732, PMID 17559755.
Chattar KB, Karve AV, Subramanyam A, Tondare SB. Prescription pattern analysis of antidepressants in psychiatric outpatient department of tertiary care hospital in India. Asian J Pharm Clin Res. 2016;9(4):77-9.
Sabu L, Yacob M, Singh H. Drug utilization pattern of psychotropic drugs in psychiatric outpatient department in a tertiary care teaching hospital. Asian J Pharm Clin Res. 2017;10(1):259-61. doi: 10.22159/ajpcr.2017.v10i1.15112.
Venkataraman R, Rayamajhi M, Islam S, NN. Prescribing pattern of psychotropic agents in rural tertiary care teaching hospital. Asian J Pharm Clin Res. 2018;11(3):155-62. doi: 10.22159/ajpcr.2018.v11i3.23063.
Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391(10128):1357-66. doi: 10.1016/S0140-6736(17)32802-7, PMID 29477251.
Wu T, Song F, Cao W, Liu C, Jia S. Comparative efficacy of antidepressant medication for adolescent depression: a network meta-analysis and systematic review. BMC Psychiatry. 2025;25(1):471. doi: 10.1186/s12888-025-06941-x, PMID 40349006.
Zhao K, Wang Y, Liu Q, Yu Z, Feng W. Efficacy comparison of five antidepressants in treating anxiety and depression in cancer and non-cancer patients. Front Neurosci. 2024 Oct 30;18:1485179. doi: 10.3389/fnins.2024.1485179, PMID 39539490.
Raju P, Qian H, Arumugham SS, Kesavan M, Wong H, YC JR. Escitalopram vs bupropion as adjunctive treatment for acute bipolar I depression: a multi-center open label trial. Int J Neuropsychopharmacol. 2025;28 Suppl 1:i120-1. doi: 10.1093/ijnp/pyae059.208, PMCID PMC11814593.
Fujii Y, Asakura S, Mitsui N. Pharmacotherapy of social anxiety disorders in children and adolescents. Int J Neuropsychopharmacol. 2025;28 Suppl 1:i105-6. doi: 10.1093/ijnp/pyae059.181, PMCID PMC11815113.
Rohde C, Hieronymus F, Ostergaard SD. A target trial emulation comparing the antidepressant effectiveness of selective serotonin reuptake inhibitors (SSRIs), highlighting the importance of patient-related confounding by indication. Acta Psychiatr Scand. 2024;150(4):198-208. doi: 10.1111/acps.13729, PMID 38994727.
Ouazana Vedrines C, Hoertel N, Lesuffleur T, Denis P, Olfson M, Blanco C. Sex differences in antidepressant acceptability according to filled prescription sequences in a nationwide cohort study. J Clin Psychiatry. 2024;85(4):23m15128. doi: 10.4088/JCP.23m15128, PMID 39630084.
Ankushe RD, Deshmukh VS, Chepure AH, Jaju JB. A comparative study of efficacy, safety and onset of action of vilazodone with escitalopram in patients of major depressive disorder at tertiary care hospital. Asian J Pharm Clin Res. 2022 Sep;15(9):113-7. doi: 10.22159/ajpcr.2022.v15i9.44812.
Kushbu D, Riyaz NM, Basha OA, Ahmed SA. Comparative study of efficacy and safety of escitalopram with escitalopram and clonazepam in patients suffering from generalized anxiety disorder a prospective randomized control study. Int J Curr Pharm Res. 2025;17(3):24-8. doi: 10.22159/ijcpr.2025v17i3.55046.