
Int J Pharm Pharm Sci, Vol 17, Issue 7, 21-24Original Article
PEDIOCIN PRODUCTION BY PEDIOCOCCUS ACIDILACTICI IN CONTINUOUS MODE USING MEAT PROCESSING WASTE
BARNALI MANDAL*
Department of Chemical Engineering, Rajabajar Science College, University of Calcutta, 92 A. P. C. Road, Kolkata-700009, West Bengal, India
*Corresponding author: Barnali Mandal; *Email: bmchemengg@caluniv.ac.in
Received: 07 Apr 2025, Revised and Accepted: 09 May 2025
ABSTRACT
Objective: The production of pediocin by Pediococcus acidilactici has been studied on a bioreactor in continuous mode. Meat processing waste (MPW) has been used as a growth medium. Different dilution rate has been attempted in continuous process to maximize the pediocin production.
Methods: The continuous fermentation process was carried out at various dilution rates, ranging from 0.075 h-1 to 0.15 h-1. A mathematical model has been developed for simulation of the continuous process.
Results: The maximum pediocin activity was observed 2743 AU/ml in continuous cultures at dilution rate of 0.075 h-1. The values of correlation coefficient (R2) were in the range of 0.94 to 0.98. The predicted model was well validated.
Conclusion: The continuous pediocin production could be possible for large-scale application.
Keywords: Pediocin, Continuous reactor, Pediococcus acidilactici, Mathematical model
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijpps.2025v17i7.55366 Journal homepage: https://innovareacademics.in/journals/index.php/ijpps
INTRODUCTION
Bacteriocins are proteinaceous compound produced by lactobacillus species show inhibition against several micro-organisms [1, 2]. Due to have antimicrobial activity, bacteriocins can be applied as an effective bio-preservative on food matrix [1, 3]. Until now, nisin and pediocin are two non-toxic bacteriocins approved as natural food additive and generally recognized as safe (GRAS) by food and drug administration (FDA) [4, 5]. Pediocin produced by Pediococcus strain have wide spectrum of inhibitory activity towards g-positive food spoiler and pathogenic bacteria such as Staphylococcus aureus, Listeria monocytogenes, Enterococcus faecalis, Clostridium perfringens etc. [5, 6]. So, pediocin could be a valuable alternative of chemical preservatives to fulfill the consumer-friendly demand with regard to health in future.
Batch fermentation has some limitations like substrate and product inhibition, low productivity, high labor cost involving in start up or shut down of reactor. The challenges of batch operation must be overcome through maximum productivity at constant dilution rate under steady state condition in continuous mode fermentation [7, 8]. In the present work, continuous pediocin production by Pediococcus acidilactici was studied under controlled pH condition and temperature at 30 °C using treated MPW growth medium in a bioreactor. Continuous fermentation process was conducted in different dilution rate to determine optimum dilution rate for highest pediocin production. A mathematical model has been developed for the simulation of continuous bioreactor. The predicted data has been compared with experimental results for the validation of mathematical model. The aim of the study is to develop large scale bioreactor for continuous pediocin production, avoiding entire difficulties of batch fermentation process.
MATERIALS AND METHODS
Microorganism and inoculum
Pediococcus acidilactici NCIM 2292 (pediocin producer strain) and indicator organigm for pediocin assay Staphylococcus aureus NCIM 2127 were procured from National Collection of Industrial Microorganisms (NCIM), National Chemical Laboratory (Pune, India) in freeze-dried form. Stock cultures were maintained as nutrient agar slants at 4 °C. S. aureus was cultivated using nutrient broth at 30 °C. MRS broth was inoculated with 2 % (v/v) culture of P. acidilactici and incubated for overnight at 30 °C. Then the strain was propagated twice in MPW broth at 30 °C before use as inoculums. The preparation of MPW broth has been described in previous work [9].
Pediocin production by continuous fermentation
The fermentation process was carried out in a 5 l stirred bioreactor (continuous mode) with working volume of 3 l having same initial conditions as in case of batch operation described in previous study [9]. The concentrations of glucose and protein in the feed were 20 g/l and 12.67 g/l, respectively. The pH was continuously monitored. The experiment was performed at different dilution rates, varying from 0.075 h-1 to 0.15 h-1. The continuous fermentation in the bioreactor was monitored by collecting samples in both transient and steady-state conditions and assaying the concentrations of biomass, pediocin, lactic acid, residual glucose and residual protein as described in previous article [9]. Each experiment was conducted in triplicate.
Model development
A mathematical model has been developed for continuous bioreactor system [10, 11] by making overall mass balance for biomass, substrates (glucose and protein) and products (pediocin and lactic acid). Where, the incoming feed is sterile. The system is perfectly stirred and hence, the concentrations of all components in the outlet stream are equal to those in the reactor. No non ideality is present in the bioreactor. The schematic of continuous bioreactor is shown in fig. 1.
Biomass
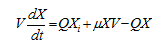 ……. (1)
……. (1)
Where, V is the reactor volume (L), Q is the effluent flow rate (L/h), microbial cell concentration is Xi (g/l) in influent and X (g/l) in effluent stream. μ is specific growth rate (h−1).
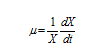
Since the feed is sterile, therefore equation (1) reduces to
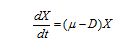 ……… (2)
……… (2)
Where, D is dilution rate (h-1).
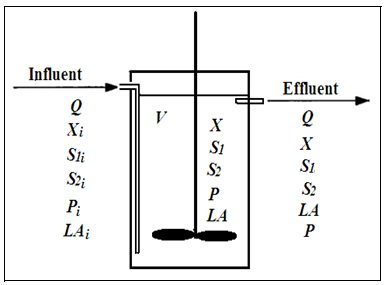
Fig. 1: Schematic of continuous fermentation process
Glucose
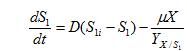 ……… (3)
……… (3)
where, S1 is concentration of glucose(g/l), S1i is glucose concentration in influent and is yield coefficient of biomass with respect to glucose.
Protein
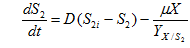 ……. (4)
……. (4)
where, S2 is protein concentration(g/l), S2i is protein concentration in influent and is yield coefficient of biomass with respect to protein.
Lactic acid
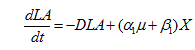 ……. (5)
……. (5)
where, LA is lactic acid concentration (g/l), α1is the growth-associated constant (g-lactic acid g-biomass-1) and β1 is the non-growth associated constant (g-lactic acid g-biomass-1h-1) for lactic acid.
Pediocin
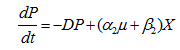 ……. (6)
……. (6)
where, P is the pediocin activity (AU/ml), α2 is growth-associated constant (AU mg-biomass-1) and β2 is the non-growth associated constant (AU mg-biomass-1 h-1) for pediocin.
Under steady state, time derivatives of all components will be zero and hence the mass balance equations will reduce to the following:
Biomass
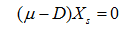 ………. (7)
………. (7)
Glucose
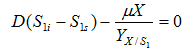 ……. (8)
……. (8)
Protein
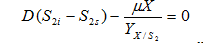 ……. (9)
……. (9)
Lactic acid
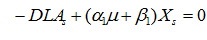 …… (10)
…… (10)
Pediocin
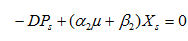 …… (11)
…… (11)
where, subscript s: at steady state
RESULTS AND DISCUSSION
In fig. 2 (a, b and c) the experimental and simulated (equations 2, 5 and 6) values of concentration of biomass, pediocin and lactic acid respectively are plotted against operating time of 5 l continuous stirred type bioreactor on respect of dilution rate (0.075-0.15 h-1). Inlet concentration of glucose and protein were 20 g/l and 12.67 g/l respectively. Similarly, the fig. 2(d and e) show graphically the both experimental and simulated (equations 3 and 4) results of glucose and protein consumption by micro-organisms varying with time. From the analysis of fig. 2 (a, b, c, d and e), it appears that steady state has been attained at 26, 30, 34 and 38 h continuous operation owing to dilution rates of 0.075 h-1, 0.1 h-1, 0.125 h-1 and 0.15 h-1 respectively. As shown in fig. 2(b), the highest pediocin activity was obtained 2743 AU/ml at dilution rate of 0.075 h-1. The values of correlation coefficient (R2) were in between 0.94 to 0.98 respectively. The agreement between simulated and experimental results is satisfactory in all cases validating the mathematical model. It further establishes the applicability of growth kinetics determined using small scale reactor, also in case of large scale bioreactor. In the insets of fig. 2 (a, b, c, d and e), the steady state concentration of biomass, pediocin, lactic acid, glucose and protein have been plotted against dilution rate. The inset fig. clearly reveal that the values of steady state concentration of products, namely, biomass, pediocin and lactic acid increase with decrease of dilution rate and in the contrary, the values of steady state concentration of glucose and protein show increasing trend with the increase of dilution rate. This is because of the fact that with increase of dilution rate, there is a reduction of residence time resulting in decrease in the extent of utilization of substrates for the conversion to biomass, pediocin and lactic acid. The value of kinetic parameters has been estimated in previous article [12].
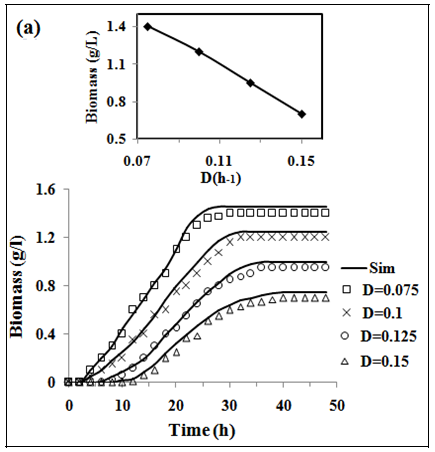
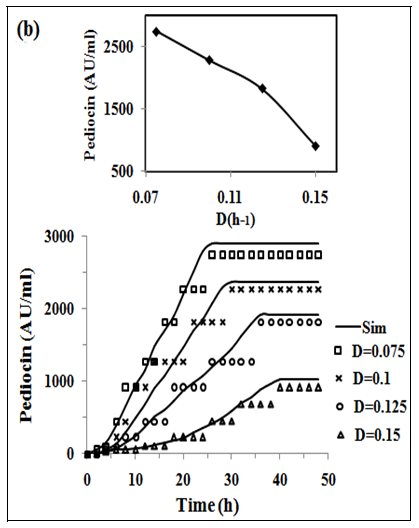
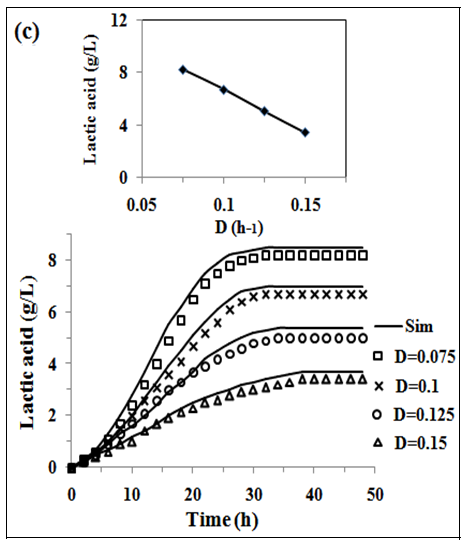
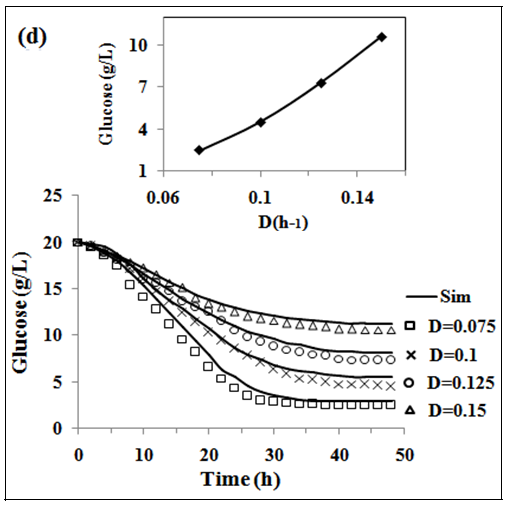
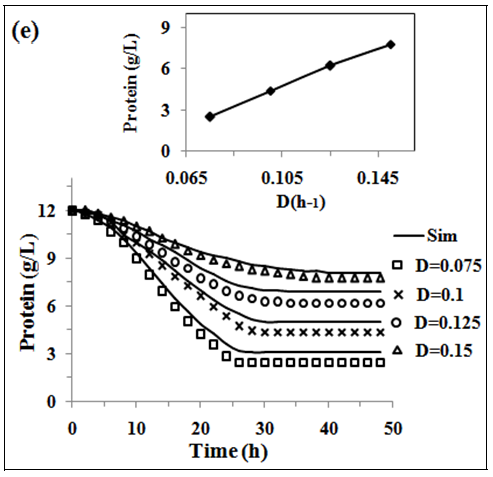
Fig. 2: (a), (b), (c), (d) and (e), experimental data of continuous fermentation of P. acidilactici NCIM 2292 on MPW medium: biomass, pediocin, lactic acid, glucose and protein at dilution rates (D) of 0.075, 0.1, 0.125 and 0.15 respectively. Continuous lines represented simulated values corresponding to the experimental results (points)
CONCLUSION
The production of pediocin by Pediococcus acidilactici was studied in continuous fermentation process using MPW medium. Pediocin was growth-associated product for continuous cultures. The continuous fermentation was conducted in varying dilution rate to determine effective dilution rate for optimal pediocin production. A mathematical model was developed to describe the bioreactor performance that could be applicable for industrial production of pediocin.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
This is author’s sole research work and not contributed by other ones.
CONFLICT OF INTERESTS
Declared none
REFERENCES
Chikindas ML, Weeks R, Drider D, Chistyakov VA, Dicks LM. Functions and emerging applications of bacteriocins. Curr Opin Biotechnol. 2018 Feb;49:23-8. doi: 10.1016/j.copbio.2017.07.011, PMID 28787641.
Cotter PD, Ross RP, Hill C. Bacteriocins a viable alternative to antibiotics? Nat Rev Microbiol. 2013;11(2):95-105. doi: 10.1038/nrmicro2937, PMID 23268227.
Mills S, Stanton C, Hill C, Ross RP. New developments and applications of bacteriocins and peptides in foods. Annu Rev Food Sci Technol. 2011;2(1):299-329. doi: 10.1146/annurev-food-022510-133721, PMID 22129385.
FDA. Nisin preparation; affirmation of GRAS status as a direct human food ingredient. Fed Registe. 1988;53:11247-51.
Bhunia AK, Johnson MC, Ray B. Purification characterization and antimicrobial spectrum of a bacteriocin produced by Pediococcus acidilactici. J Appl Bacteriol. 1988;65(4):261-8. doi: 10.1111/j.1365-2672.1988.tb01893.x, PMID 2906056.
Ming X, Weber GH, Ayres JW, Sandine WE. Bacteriocins applied to food packaging materials to inhibit Listeria monocytogenes on meats. J Food Sci. 1997;62(2):413-5. doi: 10.1111/j.1365-2621.1997.tb04015.x.
Liu X, Chung YK, Yang ST, Yousef AE. Continuous nisin production in laboratory media and whey permeate by immobilized Lactococcus lactis. Process Biochem. 2005;40(1):13-24. doi: 10.1016/j.procbio.2003.11.032.
Desjardins P, Meghrous J, Lacroix C. Effect of aeration and dilution rate on nisin Z production during continuous fermentation with free and immobilized Lactococcus lactis UL719 in supplemented whey permeate. Int Dairy J. 2001;11(11-12):943-51. doi: 10.1016/S0958-6946(01)00128-5.
Mandal B, Chowdhury R, Bhattacharjee C. Optimization of pediocin production by batch fermentation of Pediococcus acidilactici NCIM 2292 using goat meat processing waste. Res J Biotechnol. 2013;8(10):19-25.
Shuler ML, Kargi F. Bioprocess engineering basic concepts. 2nd ed; 2015 Jan 1.
Bailey JE, Ollis DF. Biochemical engineering fundamentals. 2nd ed; 1986.
Mandal B. Study the growth kinetics of Pediococcus acidilactici with estimation of kinetic parameters and applied in large scale pediocin production. Asian J Pharm Clin Res. 2016;9(5):130-5. doi: 10.22159/ajpcr.2016.v9i5.12753.