
Int J Pharm Pharm Sci, Vol 17, Issue 10, 1-6Review Article
A REVIEW ON HPLC METHOD DEVELOPMENT AND VALIDATION STUDIES OF COMBINED DOSAGE FORM OF IVACAFTOR AND TEZACAFTOR
SANDHYA MADHURI MADDALA*, JAHNAVI PARVATHI KOLA, SARIKA SUPRIYA ILLA
Department of Pharmaceutical Analysis, School of Pharmaceutical Sciences and Technologies, Jawaharlal Nehru Technological University, Kakinada, Andhra Pradesh-533003, India
*Corresponding author: Sandhya Madhuri Maddala; *Email: sandhya.munni11@gmail.com
Received: 12 Jun 2025, Revised and Accepted: 12 Aug 2025
ABSTRACT
The establishment of reliable and precise analytical methodologies plays a critical role in pharmaceutical research, manufacturing processes, and quality assurance. These methods are essential not only for identifying active pharmaceutical ingredients (APIs) but also for detecting contaminants and unintended chemical entities that may enter drug formulations during the production process. Before an analytical method can be implemented in commercial pharmaceutical manufacturing, it must be thoroughly validated by the International Council for Harmonisation (ICH) guidelines to confirm its accuracy, precision, specificity, and reliability. Cystic fibrosis (CF) is a genetic disorder affecting the lungs, digestive, and reproductive systems, caused by mutations in the CFTR gene that result in the accumulation of thick, sticky mucus leading to respiratory and digestive complications. A key therapeutic strategy for CF involves combination therapy using Tezacaftor (TZR) and Ivacaftor (IVR), two drugs that target the underlying genetic defect and improve the function of the CFTR protein. This article reviews the development and validation of analytical and bioanalytical methods for Tezacaftor and Ivacaftor, using Ultra and High-Performance Liquid Chromatography (UPLC/HPLC), and advanced techniques like LC-Tandem Mass Spectrometry (LC-MS/MS). The review outlines key steps in method development, including optimisation of chromatographic parameters and validation processes necessary to ensure that the drugs meet the required standards of purity, potency, and quality. Emphasis is placed on the importance of reliable HPLC and bioanalytical methods in supporting the production of these essential CF therapies while adhering to stringent regulatory requirements.
Keywords: Analytical method development, HPLC, Ivacaftor, Tezacaftor, Cystic fibrosis
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijpps.2025v17i10.55557 Journal homepage: https://innovareacademics.in/journals/index.php/ijpps
INTRODUCTION
Cystic fibrosis (CF) is a progressive, autosomal recessive disorder caused by mutations in the CFTR (Cystic Fibrosis Transmembrane Conductance Regulator) gene, which leads to impaired chloride ion transport across epithelial cells [1]. This results in the accumulation of thick mucus in the lungs, pancreas, and other organs, causing recurrent infections and severe respiratory and digestive complications [2]. Recent advances in CF pharmacotherapy have introduced CFTR modulators such as Ivacaftor and Tezacaftor, which significantly improve clinical outcomes by enhancing the function of the defective CFTR protein [3, 4]. The increasing clinical use of these modulators necessitates the development of highly specific, sensitive, and validated analytical methods for their accurate quantification in pharmaceutical formulations and biological matrices [5]. Techniques such as reversed-phase high-performance liquid chromatography (RP-HPLC), Ultraperformance liquid chromatography (UPLC), and Liquid chromatography-tandem mass spectrometry (LC-MS/MS) have emerged as indispensable tools for this purpose due to their precision, robustness, and high analytical throughput. Method validation must adhere to the International Council for Harmonisation (ICH) Q2(R1) guidelines, which define the essential validation parameters, including specificity, linearity, accuracy, precision, detection limit, quantitation limit, robustness, and system suitability [6]. This review critically evaluates the application of RP-HPLC, UPLC, and LC-MS/MS in the analytical method development and validation of Ivacaftor and Tezacaftor, highlighting compliance with ICH guidelines to ensure reliability, reproducibility, and regulatory acceptability in pharmaceutical quality control and therapeutic monitoring [4-6].
From 2017, initial RP-HPLC methods emerged for the simultaneous quantification of Tezacaftor and Ivacaftor in pharmaceutical formulations, validated per ICH Q2(R1) criteria [7]. By 2020, stability-indicating RP-UPLC techniques were reported, offering rapid (2 min runtime) and robust assays, again as per ICH guidelines [8]. In the field of bioanalysis, LC-MS/MS methods were developed and validated in 2021 for quantifying dual and triple-combination CFTR modulators (including Tezacaftor) in biological matrices such as plasma, sputum, and serum. These methods demonstrated high sensitivity and precision while meeting stringent EMA and FDA regulatory standards. [9This trend progressed through 2022–2024, with advanced LC‑MS/MS assays targeting small-volume samples (10–50 µl), including dried plasma spot and micro sampling formats, fully ICH-compliant and suitable for TDM in clinical settings [10]. Overall, research evolved from formulation-focused RP-HPLC to highly sensitive, matrix-specific LC-MS/MS methods, marking a robust advance in analytical validation for Tezacaftor and Ivacaftor. The main motive to select this review is due to the burning situation of Cystic Fibrosis as the people with this mutation suffer from a lot of respiratory problems and in the ease to make them relieve many more research have to be done which helps to develop more effective medication which helps in developing more effective drugs with quick relief [11]. Although LC-MS/MS has become the gold standard for bioanalysis, more sensitive and specific hyphenated techniques, such as UPLC-MS/MS and UPLC-QTOF-MS, remain underexplored for these analytes, particularly in challenging matrices like sputum and dried blood.
Drug profile
Ivacaftor
Ivacaftor, also known by its brand name Kalydeco or developmental code VX-770, is a cystic fibrosis transmembrane conductance regulator (CFTR) potentiator. It is used either as a monotherapy or in combination with other agents for the treatment of cystic fibrosis (CF) in patients who possess specific CFTR gene mutations proven to be responsive to this medication. Ivacaftor plays a key role in improving the function of defective CFTR proteins, thereby addressing the underlying cause of the disease in eligible individuals. It is manufactured and distributed by Vertex Pharmaceuticals. Ivacaftor received approval from the U. S. Food and Drug Administration (FDA) on January 31, 2012, and was subsequently approved by Health Canada later that same year. It is utilised both as a standalone therapy and in combination with other medications for the treatment of Cystic fibrosis(CF), particularly in patients with mutations in the CFTR gene that are responsive to the drug. Chemical formula: C24H28N2O3. IUPAC name for ivacaftor is N-(2,4-di-tert-butyl-5-hydroxyphenyl)-4-oxo-1,4-dihydroquinoline-3-carboxamide. Ivacaftor is practically insoluble in water, showing very low aqueous solubility. Its solubility tends to increase in organic solvents and at higher pH values, reflecting pH-dependent solubility behaviour. The compound has the strongest acidic pKa of approximately 6.57 and the strongest basic pKa around -0.95, indicating it can ionise under physiological conditions. Ivacaftor remains largely insoluble across the acidic to neutral pH range (pH 1 to 7), with improved solubility observed closer to physiological pH (around 7.4), which is significant for its absorption and formulation [12].

Fig. 1: Chemical structure of ivacaftor
Tezacftor
Tezacaftor is a cystic fibrosis transmembrane conductance regulator (CFTR) corrector medication. Developed by Vertex Pharmaceuticals, it received FDA approval for use in combination with Ivacaftor as a treatment for cystic fibrosis. This drug was approved by the FDA on February 12, 2018, with brand names Alyftrek, Kaftrio, Symdeko, Symkevi, Trikafta (100 mg/50 mg/75 mg; 150 mg). Tezacaftor in combination with Ivacaftor, is formulated as a single therapeutic product for the treatment of cystic fibrosis (CF) in individuals aged 12 years and older who either have two copies of the F508del mutation or at least one CFTR gene mutation that has been shown to respond to this medication. Chemical formula: C26H27F3N2O6. IUPAC name for Tezacaftor: 1-(2,2-Difluoro-1,3-benzodioxol-5-yl)-N-[1-[(2R)-2,3-dihydroxypropyl]-6-fluoro-2-(2-hydroxy-1,1-dimethylethyl)-1H-indol-5-yl]-cyclopropane. Tezacaftor,is a BCS Class 2 compound characterised by low solubility and high permeability. Ivacaftor has extremely low solubility in water and buffer solutions across a broad pH range (pH 1–9), with reported aqueous solubility levels below 5 µg/ml. This poor solubility creates significant challenges for oral drug delivery and absorption, particularly without formulation aids. The molecule is weakly basic, with its strongest acidic pKa at 11.54, indicating that it remains mostly neutral under physiological pH conditions. As a result, ivacaftor cannot rely on ionization to enhance solubility. In summary, Tezacaftor is practically insoluble in neutral to acidic media, exhibits negligible pH-dependent solubility improvements, and its high pKa underscores its neutral, uncharged state under biological conditions [13].
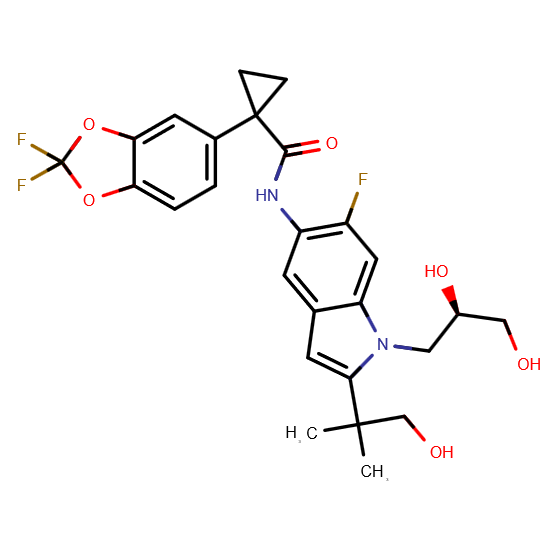
Fig. 2: Chemical structure of tezacaftor
Rama Ayyappa et al., the Kromasil C18 (150 mmx 4.6 mm, 5µm) column was used to establish a technique that maintained a flow rate of 0.7 ml/min with retention times for IVR and TZR of 2.089 and 2.434 min at a wavelength of 292 nm. The validation analysis reveals that IVR and TZR have very high correlation coefficients, at 0.994 and 0.992, respectively. IVR and TZR had recovery rates of 99.62% and 99.48%. LOD values of 0.625 µg/ml and 1.885 µg/ml, respectively, and LOQ values of 0.345 µg/ml and 1.025 µg/ml. The assay yielded 100.06% and 100.16%. Its lower flow rate suggests the method's cost-effectiveness, and according to degradation studies, acidic conditions cause more degradation [14].
Devika Subrmaniyam et al., employed a Symmetry C18 column (250 mm × 4.6 mm, 5 µm) with a mobile phase consisting of Methanol and TEA buffer (40:60 v/v, pH adjusted to 4.2). The method was optimised at a flow rate of 1.0 ml/min, yielding retention times of 2.773 min for IVR and 4.065 min for TZR, detected at 232 nm. The validation results demonstrated excellent linearity for IVR and TZR, with correlation coefficients (r²) of 0.999 each. Recoveries were 99.97% and 100.02%, respectively. The LOD and LOQ values were 0.5 µg/ml and 1.5 µg/ml for IVR, and 1.55 µg/ml and 3.5 µg/ml for TZR. Additionally, the use of a buffer reduced solvent costs in this method [15].
Lakshmi Prasanna et al., developed and validated a method using a Dionex C18 (4.6 mm x250 mm, 5µm) column with a mobile phase of 0.1MKH2PO4: Methanol (60:40v/v) at a flow rate of 1.0 ml/min and retention times of 2.397 min and 3.296 min for IVR and TZR at a wavelength of 260 nm. The validated parameters showed that the correlation coefficients (r2) for IVR and TZR were 0.997 and 0.993, and the recovery was 99.28% and 100.15%. The LOD values for IVR and TZR were 0.8 µg/ml and 2.7 µg/ml, and the LOQ values for IVR and TZR were 2.4 µg/ml and 8.3 µg/ml. In terms of degradation studies, there is greater degradation in thermal conditions as per ICHQ1A(R2), and its low mobile phase ratio suggests cost-effectiveness [16].
Narendra Singh et al., developed a technique using a Symmetry Shield RP-18 column (250 × 4.6 mm, 5 µm) with a flow rate of 1.2 ml/min. The mobile phase consisted of methanol, acetonitrile, and buffer in a ratio of 42:27:31 (v/v/v). The retention times for Ivacaftor and Tezacaftor were 3.75 min and 6.04 min, respectively, and detection was performed at a wavelength of 275 nm. The correlation coefficient (r2) for IVR and TZR was 0.999 and 0.997, respectively, and the recovery percentages were 101.13% and 99.23%. IVR and TZR have LOD values of 0.28 µg/ml and 0.77 µg/ml, respectively, and LOQ values of 0.18 µg/ml and 0.51 µg/ml. IVR and TZR assay results are 99.2%, and100.2%. They employed a buffer, which lowers the solvent costs for this process. According to degradation studies, oxidative conditions as per ICH Q1A(R2) cause higher degradation [17].
B. Balaswami et al., utilising a CHS C18 (100 x 2.1 mm, 1.7µ) column and mobile phase water: ACN (55:45v/v), an RP-UPLC technique was designed with a flow rate of 0.3 ml/min and retention times of 0.61 and 1.14 min for Ivacaftor and Tezacaftor at a wavelength of 292 nm. The validated parameter values are IVR and TZR, which have correlation coefficients(r2) of 0.995 and 0.999, respectively. LOD values for IVR and TZR are 0.30 µg/ml and 0.91 µg/ml, respectively, and LOQ values for IVR and TZR are 0.10 µg/ml and 0.31 µg/ml, with recovery rates of 99.52% and 100.61%. IVR and TZR assays are 100.06 and 100.14%, respectively. Its lower flow rate and shorter holding periods demonstrate the cost-effectiveness of the approach. Additionally, according to degradation studies, acidic environments cause higher degradation [18].
Lakshmi Menaka et al., developed a UPLC method with a CHS C18 (100 mm x 2.1 mm, 1.7µ) column and mobile phase 0.1% Ortho-phosphoric acid: ACN (50:50 v/v) at a flow rate of 0.3 ml/min. The retention times for Ivacaftor and Tezacaftor were 1.072 and 0.531 min at a wavelength of 292 nm, and the results of the validated parameters are IVR and TZR have a correlation coefficient (r2) of 0.999 and 0.999, respectively. LOQ values for IVR and TZR are 1.44µg/ml and 1.23µg/ml, respectively, and the recovery of 100.21% and 99.97%. LOD values are 0.47µg/ml and 0.41µg/ml. Its lower flow rate suggests that the analytical procedure is cost-effective. Additionally, according to degradation studies, acidic environments cause higher degradation [8].
Shyamala et al., used an HSS C18 (100 mm x 2.1 mm,1.7µ) column with mobile phase 0.1% orthophosphoric acid buffer: ACN (50:50 v/v) with a flow rate of 0.3 ml/min and retention times of 0.514 min and 0.944 min, at a wavelength of 292 nm. The results of the parameter validation are IVR and TZR have a correlation coefficient (r2) of 0.999 and 0.999, respectively. The method demonstrated excellent recovery rates of 99.97% for IVR and 99.65% for TZR, with LOD values of 0.41 µg/ml (IVR) and 0.12 µg/ml (TZR). The corresponding LOQ values were 1.24 µg/ml (IVR) and 0.37 µg/ml (TZR). Assay results further confirmed high accuracy, yielding 99.85% for IVR and 100.02% for TZR. The method's cost-effectiveness is highlighted by its low flow rate, short retention times, and reduced buffer consumption. Additionally, degradation studies conducted in compliance with ICH Q1A (R2) guidelines revealed that both compounds undergo greater degradation under acidic conditions [19].
M. Satya Venkata Sakuntala et al., developed a simple, sensitive, accurate, robust LC-MS/MS method for the simultaneous quantification of IVR and TZR in rat plasma using Eclipse plus C18 analysis column (100 mm × 4.6 mm, 1.8 µm) with a mobile phase of 0.1% trifluoroacetic acid: acetonitrile (60:40 % v/v, and pH 2.5) at a flow stream of 1.0 ml/min at room temperature for bioanalytical validation using liquid chromatography coupled with tandem-mass spectrometry.50 µl had been the injection volume for the sample. As internal standards, deuterated (d4-substituted) ivacaftor-d4 and tezacaftor-d4 were used. The LLOQ values for IVR and TZR for system suitability are 1.5 and 1.0 ng/ml, respectively. IVR and TZR recovery rates were 99.58% and 99.45%, respectively. % CV for TZR and IVR were 3.444 and 4.229. Ivacaftor and Tezacaftor's linearity was assessed in the ranges of 1.5 ng/ml to 22.53 ng/ml and 1 ng/ml to 15.02 ng/ml, respectively. The USFDA requirements are followed when conducting degradation studies [20].
Table 1: Forced degradation study conditions of Ivacaftor and Tezacaftor for HPLC and UPLC
| Stress condition | Degradation type | Experimental conditions | Purpose | Reference |
| Acidic hydrolysis | Hydrolytic | 0.1N HCl, 60 °C for 2–4 h | Assess stability in gastric-like conditions | [8] |
| Basic hydrolysis | Hydrolytic | 0.1N NaOH, 60 °C for 2–4 h | Simulate alkaline hydrolysis risk | |
| Oxidative stress | Oxidative | 3% H₂O₂ at room temperature for 2–6 h | Evaluate sensitivity to oxidative environments | |
| Thermal degradation | Thermal | 105 °C dry heat exposure for 24–48 h | Test thermal stability during storage | |
| Photolytic degradation | Photolytic (UV/Visible) | UV light (1.2 million lux hours), 200 W/m² for 48 h | Assess light sensitivity | |
| Humidity stress | Hydrolytic/Moisture | 90% RH at 40 °C for 7 d | Evaluate impact of high humidity |
Table 2: Degradation studies of ivacaftor and tezacaftor
| RP-HPLC | |||
| Rama Ayyapa et al., [14] | |||
| Degradation | Ivacaftor | Tezacaftor | |
| Acid | 6.71 | 7.20 | |
| Base | 5.95 | 7.97 | |
| Peroxide | 2.95 | 6.35 | |
| Thermal | 2.02 | 6.39 | |
| UV | 1.46 | 1.56 | |
| Neutral | 1.46 | 0.8 | |
| Laxmi Prasanna M. et al., [16] | |||
| Acid | 5.22 | 3.97 | |
| Base | 3.80 | 2.84 | |
| Peroxide | 2.70 | 2.18 | |
| Thermal | 6.57 | 4.70 | |
| Sun light | 5.02 | 2.02 | |
| Water | 1.09 | 0.79 | |
| Narendra Singh et al., [17] | |||
| Acid | 15.59 | 17.78 | |
| Base | 5.13 | 9.82 | |
| Oxidative | 20.06 | 28.35 | |
| Thermal | 1.42 | 2.12 | |
| Photodegradation | 1.22 | 1.51 | |
| RP-UPLC | |||
| Lakshmi Menakaet al., [8] | |||
| Acid | 7.84 | 8.41 | |
| Base | 6.44 | 7.18 | |
| Peroxide | 6.81 | 5.18 | |
| Dry heat | 2.02 | 3.40 | |
| Photostability | 1.54 | 2.12 | |
| Water sample | 0.49 | 0.86 | |
| B Balaswamiet al.,[18] | |||
| Acid | 5.09 | 5.75 | |
| Base | 4.45 | 4.84 | |
| Peroxide | 3.28 | 3.88 | |
| Thermal | 2.96 | 2.49 | |
| UV | 1.86 | 1.96 | |
| Water | 1.86 | 0.83 | |
| Shyamala et al.,[20] | |||
| Acid | 4.86 | 8.78 | |
| Base | 3.14 | 5.93 | |
| Peroxide | 3.06 | 3.36 | |
| Thermal | 2.99 | 2.69 | |
| UV | 1.13 | 1.06 | |
| Water | 0.73 | 0.44 | |
Table 3: Bio-analytical forced degradation for LC-MS/MS
| Required stability tests per U. S. FDA (Analogous to forced degradation) | References | ||
| Stability Type | Purpose | Typical Conditions | [21] |
| Short-term Stability | Sample handling | Room temp (4–24 h) | |
| Long-term Stability | Storage stability | -20 °C,-80 °C for weeks/months | |
| Freeze-Thaw Stability | Repeated freezing cycles | ≥3 cycles | |
| Post-Preparative Stability | Autosampler or bench stability | 4–24 h at autosampler conditions | |
| Stock Solution Stability | Analyte standard and internal standard stability | RT and refrigerated |
Table 4: LC-MS/MS degradation studies for ivacaftor and tezacaftor ivacaftor
| Stability | Mean peak of stability sample | %CV | Mean peak at zero time | %CV | % Deviation | References |
| Auto sampler stability | ||||||
| LQC | 1.04 | 1.02 | 1.02 | 1.16 | 1.66 | [20] |
| MQC | 1.68 | 1.68 | 2.01 | 1.02 | 1.18 | |
| HQC | 1.26 | 1.26 | 3.02 | 1.20 | 1.11 | |
| Short term stability | ||||||
| LQC | 0.989 | 0.26 | 0.986 | 0.28 | 0.30 | [20] |
| HQC | 2.989 | 0.24 | 2.985 | 0.21 | 0.13 | |
| Freeze Thaw Stability | ||||||
| LQC | 1.013 | 1.23 | 1.005 | 1.28 | 0.79 | [20] |
| HQC | 3.011 | 0.10 | 3.014 | 0.18 | -009 | |
| LT at-20 °C | ||||||
| LQC | 0.766 | 1.46 | 0.752 | 1.21 | 1.86 | [20] |
| HQC | 2.786 | 0.18 | 2.781 | 0.15 | 0.18 | |
| LTat-80 °C | ||||||
| LQC | 0.687 | 0.23 | 0.699 | 1.28 | -0.43 | [20] |
| HQC | 2.682 | 0.40 | 2.679 | 0.49 | 0.11 |
Table 5: Tezacaftor
| Stability | Mean peak of stability sample | %CV | Mean peak at zero time | %CV | % Deviation | References |
| Auto sampler stability | ||||||
| LQC | 0.753 | 1.52 | 0.751 | 1.28 | 0.26 | [20] |
| MQC | 1.507 | 1.75 | 1.504 | 1.65 | 0.19 | |
| HQC | 2.255 | 1.57 | 2.251 | 1.45 | 0.17 | |
| Short term stability | ||||||
| LQC | 0.710 | 0.65 | 0.712 | 0.68 | -0.28 | [20] |
| HQC | 2.2213 | 0.29 | 2.205 | 0.61 | 0.36 | |
| Freeze thaw stability | ||||||
| LQC | 0.754 | 1.35 | 0.751 | 1.18 | 0.39 | [20] |
| HQC | 2.255 | 0.98 | 2.256 | 0.62 | -0.04 | |
| Long term stability at-20 °C | ||||||
| LQC | 0.492 | 0.79 | 0.491 | 0.44 | 0.20 | [20] |
| HQC | 1.939 | 0.54 | 1.932 | 1.21 | 0.36 | |
| Long term stabilityat-80 °C | ||||||
| LQC | 0.389 | 1.59 | 0.384 | 1.64 | 1.30 | [20] |
| HQC | 1.866 | 0.87 | 1.868 | 1.14 | -0.10 |
Table 6: Chromatographic conditions and results of tezacaftor and ivacaftor [14-20]
| Parameter | Rama Ayyappa et al. | Devika Subrmaniyam et al. | Lakshmi Prasanna et al. | Narendra Singh et al. | B. Balaswami et al. | Lakshmi Menaka et al. | Shyamala et al. |
| Reference | [14] | [15] | [16] | [17] | [18] | [8] | [20] |
| Column | Kromasil C18 (150 × 4.6 mm, 5 µm) | Symmetry C18 (250 × 4.6 mm, 5 µm) | Dionex C18 (250 × 4.6 mm, 5 µm) | Symmetry Shield RP18 (250 × 4.6 mm, 5 µm) | CHS C18 (100 × 2.1 mm, 1.7 µm) | CHS C18 (100 × 2.1 mm, 1.7 µm) | HSS C18 (100 × 2.1 mm, 1.7 µm) |
| Mobile Phase | Water: ACN (50:50 v/v) | Methanol: TEA buffer (pH 4.2, 40:60 v/v) | 0.1M KH₂PO₄:Methanol (60:40 v/v) | Buffer: Methanol: ACN (42:27:31 v/v) | Water: ACN (55:45 v/v) | 0.1% OPA: ACN (50:50 v/v) | 0.1% OPA buffer: ACN (50:50 v/v) |
| Flow rate (ml/min) | 0.7 | 1.0 | 1.0 | 1.2 | 0.3 | 0.3 | 0.3 |
| Retention time (min) | IVR: 2.089 TZR: 2.434 |
IVR: 2.773 TZR: 4.065 |
IVR: 2.397 TZR: 3.296 |
IVR: 3.75 TZR: 6.04 |
IVR: 0.617 TZR: 1.146 |
IVR: 1.072 TZR: 0.531 |
IVR: 0.514 TZR: 0.944 |
| Detection wavelength (nm) | 292 | 232 | 260 | 275 | 292 | 292 | 292 |
| Correlation coefficient (r²) | IVR: 0.994 TZR: 0.992 |
IVR: 0.9999 TZR: 0.999 |
IVR: 0.997 TZR: 0.993 |
IVR: 0.999 TZR: 0.997 |
IVR: 0.995 TZR: 0.999 |
IVR: 0.999 TZR: 0.999 |
IVR: 0.999 TZR: 0.999 |
| % Recovery | IVR: 99.62 TZR: 99.48 |
IVR: 99.97 TZR: 100.02 |
IVR: 99.28 TZR: 100.15 |
IVR: 101.14 TZR: 99.23 |
IVR: 99.52 TZR: 100.61 |
IVR: 100.21 TZR: 99.97 |
IVR: 99.97 TZR: 99.68 |
| LOD (µg/ml) | IVR: 0.625 TZR: 1.885 |
IVR: 0.5 TZR: 1.5 |
IVR: 0.8 TZR: 2.7 |
IVR: 0.28 TZR: 0.77 |
IVR: 0.30 TZR: 0.10 |
IVR: 0.47 TZR: 1.44 |
IVR: 0.41 TZR: 0.12 |
| LOQ (µg/ml) | IVR: 0.345 TZR: 1.025 |
IVR: 1.55 TZR: 1.5 |
IVR: 1.4 TZR: 1.36 |
IVR: 0.18 TZR: 0.51 |
IVR: 0.91 TZR: 0.31 |
IVR: 1.44 TZR: 1.23 |
IVR: 1.24 TZR: 0.37 |
| Precision (% RSD) | Method Precision IVR: 0.5–0.7 TZR: 0.2–0.5 Intermediate Precision IVR: 0.3–0.9 TZR: 0.6–0.8 |
Method Precision IVR: 0.125 TZR: 0.362 Intermediate Precision IVR: 0.553 TZR: 0.472 |
Method Precision IVR: 0.163 TZR: 0.845 |
Method Precision IVR: 0.29 TZR: 0.39 Intermediate Precision IVR: 1.18 TZR: 1.02 |
Method Precision IVR: 0.5 TZR: 0.9 |
Method Precision IVR: 0.9 TZR: 0.9 Intermediate Precision IVR: 0.8 TZR: 1.1 |
Method Precision IVR: 0.5 TZR: 0.2 |
| Assay (%) | IVR: 100.06 TZR: 100.16 |
IVR: 99.97 TZR: 49.98 |
– | IVR: 99.82 TZR: 100.21 |
IVR: 100.06 TZR: 100.14 |
– | IVR: 99.85 TZR: 100.02 |
Abbreviations: LOD = Limit of Detection, LOQ = Limit of Quantification, OPA = Orthophosphoric Acid, ACN = Acetonitrile, TEA = Triethylamine.
Importance of analytical method
To ensure the safety and efficacy of pharmaceutical formulations, an analytical approach must effectively identify not only the active ingredient but also all potential chemical species arising from manufacturing processes, storage conditions, and stress factors. Detecting impurities beyond the active pharmaceutical ingredient (API) and excipients is critical. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) is a highly sensitive and precise technique capable of detecting and quantifying all chemical components within a complex mixture, making it an essential tool for comprehensive bioanalytical assessment. UPLC is a sensitive and precise method capable of detecting all chemical components within a mixture. Therefore, selecting the most appropriate analytical approach to address specific challenges necessitates a thorough understanding of various techniques. Existing HPLC, UPLC, and LC-MS/MS methods have been employed to examine Ivacaftor and Tezacaftor together as a combined drug formulation. The current study focuses on the development and analytical validation of different HPLC and UPLC methods for Tezacaftor and Ivacaftor and has been designed by ICH guidelines.
CONCLUSION
The literature highlights the development of an analytical technique using Reverse-Phase Liquid Chromatography (RP-LC), Ultra Performance Liquid Chromatography (UPLC), and Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS)methods well-suited for both molecular and bioanalytical analysis. Given the high polarity of the compounds, reverse-phase chromatography facilitates the early elution of the active molecules. This enables Ivacaftor (IVR) and Tezacaftor (TZR) to elute within 2–6 min in conventional HPLC, while UPLC further reduces the runtime to just 0.51–1.14 min, enhancing efficiency for commercial laboratories and production facilities. The LC-MS/MS-based bioanalytical method developed for IVR and TZR in rat plasma proved to be rapid, simple, and robust, making it suitable for routine bioanalytical research. Further insights into method optimization, tailored to different laboratory needs, whether analyzing Tezacaftor and Ivacaftor individually or in combination, will be discussed in the review. Additionally, stability studies indicate that these analytes undergo greater degradation under acidic, thermal, and oxidative conditions compared to other stress factors. The described methods are recognized for their precision and efficiency in assessing drug stability and quality.
FUNDING
This review article has no funding.
AUTHORS CONTRIBUTIONS
Sandhya Madhuri Maddala contributed to the conceptualization of the study, methodology design, data curation, critical review of analytical methods, and validation of information, supervision, project administration, and final manuscript approval. She also provided essential resources for the research. Kola Jahnavi Parvathi conducted the literature review, prepared the original draft, and participated in writing, reviewing, and editing the manuscript. Illa Sarika Supriya was responsible for compiling and analyzing drug profiles. All authors have thoroughly reviewed and approved the final version of the manuscript. Each contributor played a significant role in this work and assumes full responsibility for its content.
CONFLICTS OF INTEREST
The authors confirm that there are no conflicts of interest related to this work.
REFERENCES
Riordan JR. CFTR function and prospects for therapy. Annu Rev Biochem. 2008;77:701-26. doi: 10.1146/annurev.biochem.75.103004.142532, PMID 18304008.
O Sullivan BP, Freedman SD. Cystic fibrosis. Lancet. 2009 May 30;373(9678):1891-904. doi: 10.1016/S0140-6736(09)60327-5, PMID 19403164.
Ramsey BW, Davies J, Mc Elvaney NG, Tullis E, Bell SC, Drevinek P. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365(18):1663-72. doi: 10.1056/NEJMoa1105185, PMID 22047557.
Taylor Cousar JL, Munck A, McKone EF, Van Der Ent CK, Moeller A, Simard C. Tezacaftor ivacaftor in patients with cystic fibrosis homozygous for Phe508del. N Engl J Med. 2017 Nov 23;377(21):2013-23. doi: 10.1056/NEJMoa1709846, PMID 29099344.
Donakonda M, Indrakanti S, Pasala PK, Desari M, Kammari S. A rapid RP-HPLC method for the simultaneous estimation of ivacaftor and tezacaftor and in silico study of their metabolitic products. Futur J Pharm Sci. 2021;7(1):2-14. doi: 10.1186/s43094-021-00254-y.
ICH Harmonised Tripartite Guideline. Validation of analytical procedures: text and methodology Q2(R1). International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH); 2005.
Kiranjyothi R, Balakrishnan M, Chandrasekhar KB. Method development and validation for the stability indicating simultaneous estimation of Tezacaftor and ivacaftor in bulk and its dosage forms. Future J Pharm Sci; 2018.
Maneka SL, Saravanakumar RT, Anjana CH, Anjana CHK VLSN. Development and validation of stability indicating RP‑UPLC method for the simultaneous estimation of tezacaftor and ivacaftor in formulations. Int J Pharm Pharm Sci. 2020;12(9):63-70. doi: 10.22159/ijpps.2020v12i9.38412.
Vonk SE, Van Der Meer Vos M, Bos LD, Neerincx AH, Majoor CJ, Maitland Van Der Zee AH. Quantitative method for the analysis of ivacaftor hydroxymethyl ivacaftor ivacaftor carboxylate lumacaftor and tezacaftor in plasma and sputum using liquid chromatography with tandem mass spectrometry and its clinical applicability. Ther Drug Monit. 2021;43(4):555-63. doi: 10.1097/FTD.0000000000000829, PMID 33165217.
Zheng Y, Rouillon S, Khemakhem M, Balakirouchenane D, Lui G, Abdalla S. A rapid LC-MS/MS method for the simultaneous quantification of ivacaftor, lumacaftor elexacaftor tezacaftor hexyl-methyl ivacaftor and ivacaftor carboxylate in human plasma. J Pharm Biomed Anal. 2024 Sep 15;248:116322. doi: 10.1016/j.jpba.2024.116322, PMID 38964167.
Habler K, Kalla AS, Rychlik M, Bruegel M, Teupser D, Nahrig S. Isotope dilution LC-MS/MS quantification of the cystic fibrosis transmembrane conductance regulator (CFTR) modulators ivacaftor lumacaftor, tezacaftor, elexacaftor and their major metabolites in human serum. Clin Chem Lab Med. 2022 Jan 26;60(1):82-91. doi: 10.1515/cclm-2021-0724, PMID 34668357.
Drug Bank. Ivacaftor. Available from: www.https://go.drugbank.com/drugs/db08820.
Drugbank T. Drug Bank. Available from: https://gocom/drugs/db11712. [Last accessed on 12 Apr 2025].
Rama Ayyappa M, Raveendra Babu GR, Sushama C, Sowjanya M, Lakshmana Murthy G. Development and validation of a novel stability indicating RP-HPLC method for the simultaneous estimation of tezacaftor and ivacaftor in combined tablet dosage form. J Chromatogr Sci. 2020 Jun;58(6):456-62. doi: 10.1093/chromsci/58.6.456, PMID 32470108.
Devikasubramaniyan G, Rajendran R, Anandhakumar C. A novel RP‑HPLC method for the estimation of ivacaftor and tezacaftor in bulk and pharmaceutical dosage formulations. Int J Pharm Anal Res. 2023 Jan-Mar;12(1):9-15. doi: 10.61096/ijpar.v12.iss1.2023.9-15.
Laxmi Prasanna M, Bakshi A, Bhagavan Raju M. New RP‑HPLC method development and validation for simultaneous estimation and forced degradation studies of ivacaftor and tezacaftor in solid dosage form. World J Pharm Res. 2019 Dec;8(13):913-22. doi: 10.20959/wjpr201913‑15977.
Singh N, Bansal P, Maithani M, Chauhan Y. Development and validation of a novel stability indicating RP-HPLC method for simultaneous determination of tezacaftor and ivacaftor in fixed dose combination. J Chromatogr Sci. 2020 Apr;58(4):346-54. doi: 10.1093/chromsci/bmz120, PMID 31953544.
Balaswami B, Venkata Ramana P. A new stability-indicating RP‑UPLC method development and validation for the simultaneous estimation of ivacaftor and tezacaftor in the pharmaceutical dosage form. Int J Pharm Biol Sci. 2019;9(4):1158-66. doi: 10.21276/ijpbs.2019.9.3.143.
Shyamala DA. A novel stability-indicating UPLC method for the estimation of tezacaftor and ivacaftor in tablet dosage form. Int J Pharm Sci Res. 2019 Nov;10(11):4968-73. doi: 10.13040/IJPSR.0975-8232.10(11).4968-73.
Venkata MS, Rao AL, Carey MW. Simultaneous estimation of ivacaftor and tezacaftor in rat plasma by liquid chromatography coupled with tandem mass-spectrometry: application to pharmacokinetic studies. Thai J Pharm Sci. 2021;45(6):451-60. doi: 10.56808/3027-7922.2526.
Sutar SV, Yeligar VC, Patil SS. A review: stability indicating forced degradation studies. Res J Pharm Technol. 2019;12(2):885-90. doi: 10.5958/0974-360X.2019.00152.5.
Indira Priyadarshini G, Mounika V, Anjani G, Sowmya B. Stability indicating RP‑HPLC method development and validation for the simultaneous estimation of tezacaftor and ivacaftor in bulk and pharmaceutical dosage form. Asian J Pharm Anal. 2020;10(1):19-26. doi: 10.5958/2231‑5675.2020.00005.
Marakatham S, Shanmugapandiyan P. Stability indicating bioanalytical validation of elexacaftor ivacaftor and tezacaftor using HPLC in human plasma. Res J Pharm Technol. 2023;16(8):3780-6. doi: 10.52711/0974-360X.2023.00624.
Alle S, Ubbani R, Gangadi JR, Tomar S, Kokkula PK. Ensuring stability and accuracy: bioanalytical validation of elexacaftor ivacaftor and tezacaftor in human plasma by HPLC analysis. J Chem Pharm Sci. 2024;17(1):18-27.
Zehra F, Begum KA, Ramakrishna D. Method development and validation of simultaneous estimation of tezacaftor and ivacaftor in API by RP-HPLC. Int J Pharm Ind Res. 2021;11(2):18-27.
Pavani K, Sravanasree B, Vineela M. A new RP-HPLC method for the determination of tezacaftor and ivacaftor in bulk form and marketed pharmaceutical dosage form. Indo Am J Pharm Sci. 2023 Jul;10(7):122-34. doi: 10.5281/zenodo.8206048.
Patel D, Patel J, Patel N. Development and validation of a stability indicating RP-HPLC method for simultaneous estimation of paracetamol and diclofenac potassium in bulk and combined dosage form. Int J Curr Pharm Rev Res. 2019;10(3):1-7.
Kumar V, Sharma V, Singh S. Simultaneous estimation of metformin hydrochloride and sitagliptin phosphate monohydrate by UV spectrophotometric method in tablet dosage form. Int J Appl Pharm. 2017;9(5):29-34. doi: 10.22159/ijap.2017v9i5.20578.
Choudhary D, Kaur N, Singh S. Simultaneous spectrophotometric estimation of amlodipine besylate and valsartan in pharmaceutical dosage form. Asian J Pharm Clin Res. 2018;11(7):1-5. doi: 10.22159/ajpcr.2018.v11i7.25051.
Singh P, Singh K, Singh S. Development and validation of simultaneous estimation of rabeprazole sodium and domperidone by UV spectrophotometric method in combined dosage form. Int J Curr Pharm Rev Res. 2018;9(4):45-51.
Kumar A, Sharma S, Saini S. RP-HPLC method development and validation for simultaneous estimation of paracetamol and aceclofenac in tablet dosage form. Asian J Pharm Clin Res. 2019;12(10):233-7. doi: 10.22159/ajpcr.2019.v12i10.33038.