
Int J Pharm Pharm Sci, Vol 17, Issue 9, 1-7Review Article
SUSTAINABILITY AND QUALITY IN THE PHARMACEUTICAL INDUSTRY: THE CRITICAL ROLE OF QUALITY ASSURANCE IN GREEN MANUFACTURING AND ENVIRONMENTAL STEWARDSHIP
VINEETH RAJ K., RAVEESH GOPAL HEGDE, ANOOP NARAYANAN V.*
Nitte (Deemed to be University), NGSM Institute of Pharmaceutical Sciences (NGSMIPS), Department of Pharmaceutical Quality Assurance, Deralakatte, Mangalore, Karnataka-575018, India
*Corresponding author: Anoop Narayanan V.; *Email: anoopnarayanan@nitte.edu.in
Received: 17 Jun 2025, Revised and Accepted: 26 Jul 2025
ABSTRACT
The pharmaceutical industry, while essential to public health, contributes significantly to environmental degradation through energy-intensive processes, hazardous waste, and high carbon emissions. This review explores the evolving role of quality assurance (QA) as a strategic enabler of sustainability within pharmaceutical manufacturing. Traditionally focused on compliance and product integrity, QA is now expanding to incorporate green chemistry principles, environmental auditing, and sustainability metrics into quality systems. The integration of digital tools such as Digital Quality Management Systems (QMS), Artificial Intelligence (AI), and the Internet of Things (IoT), under the Pharma 4.0 framework, is transforming QA into a real-time, data-driven pillar of sustainable operations. The review emphasizes the implementation of life cycle assessments (LCA), eco-design strategies, renewable feedstocks, solvent recovery, and green documentation practices across the product lifecycle from R&D to distribution and disposal. It further highlights the importance of cross-functional collaboration, regulatory alignment, and stakeholder engagement in achieving green objectives. Despite promising innovations, the study identifies several barriers, including regulatory inflexibility, a lack of standardized green QA frameworks, and resistance to organizational change. Strategic enablers, including total quality management (TQM), employee empowerment, policy incentives, and environmental governance, are discussed as critical to imgding sustainability in quality systems. Ultimately, QA's alignment with environmental stewardship not only ensures regulatory compliance and operational efficiency but also fosters long-term corporate resilience and societal trust. By imgding sustainability into the pharmaceutical quality framework, this transformation supports the industry’s shift toward greener, more ethical, and future-ready manufacturing practices that align with global environmental and public health goals.
Keywords: Sustainability, Pharmaceutical industry, Quality assurance (QA), Green chemistry, Pharma 4.0, Environmental stewardship, Life cycle assessment (LCA), Digital quality management, GMP, Total quality management (TQM)
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijpps.2025v17i9.55633 Journal homepage: https://innovareacademics.in/journals/index.php/ijpps
INTRODUCTION
The global pharmaceutical industry, while pivotal in advancing public health, faces growing scrutiny for its environmental impact [1-3]. The rising urgency of climate change, resource depletion, and ecological degradation has spurred international efforts like the 2030 Agenda for Sustainable Development by the United Nations, which outlines 17 Sustainable Development Goals (SDGs) that call for responsible consumption, industrial innovation, and urgent climate action. Within this global framework, the pharmaceutical sector is challenged not only to provide safe and effective medicines but also to reduce its environmental footprint [4-6].
Pharmaceutical manufacturing processes are resource-intensive, often involving hazardous chemicals, high energy consumption, and significant waste generation. A 2019 study found that the pharmaceutical industry emits approximately 48.55 tons of CO₂-equivalent per $1 million in revenue, surpassing even the automotive industry by 55%. This dual responsibility, ensuring patient safety and promoting environmental stewardship, becomes central to sustainable pharmaceutical practices [7, 8]. Moreover, a recent comparative analysis of sustainable manufacturing across industries reveals that pharmaceuticals still lag in adopting lean, low-emission production models, necessitating accelerated adoption of cross-sector green technologies [9-13].
Table 1: Carbon footprint comparison of industries
| Industry | Carbon emission (tons CO₂e per $1 million revenue) | Source |
| Pharmaceutical | 48.55 | [9, 10] |
| Automotive | 31.40 | |
| Semiconductor | 20.50 | |
| Textiles | 35.60 |
Compared to other sectors, the pharmaceutical industry exhibits a notably higher carbon footprint, reinforcing the need for adopting greener practices. Table 1 illustrates the comparative emissions among key industries, and the source of this data is mentioned in the table as [9, 10].
Traditionally, quality assurance (QA) has functioned as a compliance-driven system to ensure that medicines meet safety, efficacy, and regulatory requirements. However, with increasing environmental concerns and regulatory pressure, QA is evolving into a strategic enabler of sustainability [14, 15]. By integrating sustainability into QA frameworks, organizations can align quality systems with green chemistry principles, energy-efficient operations, and waste minimization protocols. This evolution expands QA’s role from a gatekeeper of compliance to a proactive architect of sustainable development in the industry.
The rationale for this review is to explore how QA can bridge the gap between conventional pharmaceutical quality standards and sustainable manufacturing practices. While significant progress has been made in applying green chemistry and lean manufacturing tools, a structured QA perspective that imgs sustainability into each stage of the product lifecycle from R and D to distribution remains underexplored [16, 17]. In particular, aligning QA with material sustainability and life-cycle thinking enhances the evaluation of long-term environmental trade-offs, fostering a cradle-to-grave accountability framework [18, 19].
Hence, this review aims to provide a critical analysis of QA's role in enabling sustainable pharmaceutical manufacturing, focusing on green initiatives, regulatory integration, quality metrics, and long-term environmental responsibility. This review was conducted through structured literature searches using databases such as PubMed, ScienceDirect, and Scopus. The search used keywords including “pharmaceutical sustainability”, “green chemistry”, “quality assurance in pharma”, “environmental stewardship”, and “Pharma 4.0”. Articles published from 2015 to 2024 were included, with emphasis on peer-reviewed studies and guidelines from regulatory agencies. Non-English and non-peer-reviewed materials were excluded.
Green pharmaceutical manufacturing: context and drivers
The pharmaceutical industry is undergoing a major transition, driven by mounting pressure to reduce its ecological footprint. Conventional manufacturing practices have historically been linear, energy-intensive, and heavily reliant on non-renewable resources. These operations contribute significantly to carbon emissions, hazardous waste generation, and water consumption, all of which undermine environmental sustainability [7, 11, 66].
One of the primary catalysts for this transformation is regulatory pressure combined with public and investor demand for sustainable practices. For instance, guidelines from regulatory bodies such as the U. S. Environmental Protection Agency (EPA), European Medicines Agency (EMA), and the World Health Organization (WHO) are increasingly aligned with green manufacturing goals, urging pharmaceutical companies to adopt cleaner production techniques and green chemistry. Initiatives such as the EU (European Union) Green Deal and the FDA’s modernization plan also emphasize reducing emissions, transitioning to circular economy models, and integrating life cycle thinking into drug production [16].
Green pharmaceutical manufacturing integrates principles of green chemistry and green engineering into drug development and production processes. These principles emphasize reducing or eliminating the use of hazardous substances, optimizing energy efficiency, minimizing waste, and maximizing atom economy [20, 67]. Core concepts such as solvent minimization, catalysis over stoichiometric reagents, and use of safer, renewable feedstocks are increasingly applied across the product life cycle [16]. Pharmaceutical firms are also exploring energy benchmarking and emission forecasting tools that have shown success in other manufacturing sectors, offering a roadmap for broader implementation [13].
Table 2: Green chemistry principles in pharma manufacturing
| Principle | Application in pharma manufacturing | Example | Source |
| Prevent Waste | Optimize synthesis to minimize waste | Continuous manufacturing methods | [23] |
| Safer Solvents and Auxiliaries | Use safer, less toxic solvents | Supercritical CO₂ instead of organic solvents | |
| Energy Efficiency | Conduct reactions at ambient temperature/pressure | Catalytic reactions replacing heating steps | |
| Use of Renewable Feedstocks | Use bio-based or renewable starting materials | Fermentation-derived APIs | |
| Design for Degradation | Design drugs to break down safely in the environment | Biodegradable drug compounds |
The application of green chemistry principles enables pharmaceutical industries to achieve eco-friendly production goals. Table 2 summarizes important principles and their relevance to pharmaceutical manufacturing [23].
Companies are now moving toward solvent recovery, water reuse, waste valorization, and implementing closed-loop systems as part of their environmental sustainability initiatives [14]. A growing number of firms are adopting environmental management systems (EMS) aligned with ISO 14001 to track and reduce environmental burden [21, 22].
Stakeholder expectations are also reshaping the industry’s sustainability agenda. ESG-focused investors, climate-smart healthcare providers, and government procurement bodies are increasingly favoring companies with strong green credentials [21]. These expectations drive transparency, accountability, and innovation in sustainable production. Pharmaceutical companies are aligning their corporate social responsibility (CSR) strategies with global frameworks like the Paris Agreement and Sustainable Development Goals (SDGs) to decarbonize supply chains and contribute to broader climate objectives [4, 21].
Market competitiveness and cost-effectiveness are also critical drivers. Adopting sustainable practices can lead to reduced raw material costs, improved process efficiency, and lower waste management expenses. More importantly, green credentials have become a reputational asset, influencing consumer behaviour and investor decisions [20]. Recent studies highlight the potential of modular, scalable green manufacturing units to reduce energy costs while improving agility and carbon tracking in pharma supply chains [25].
Quality assurance in the age of sustainability
Traditionally, Quality assurance (QA) in the pharmaceutical industry has centered on ensuring that products meet regulatory standards related to good manufacturing practices (GMP), product safety, efficacy, and compliance. QA systems are designed to maintain consistency in manufacturing, conduct documentation and batch review, and ensure traceability throughout the product lifecycle [26]. However, the evolving global focus on sustainability has necessitated a paradigm shift in the scope and responsibilities of QA.
Modern QA is no longer confined to conventional quality metrics but is being reshaped into a governance model that includes environmental accountability. This expansion is catalyzed by increasing regulatory expectations, ESG (Environmental, Social, Governance) imperatives, and the industry’s commitment to green transformation. As sustainability becomes a strategic priority, QA is taking a more proactive role in enabling and verifying sustainable practices across the manufacturing ecosystem [14].
One of the core expansions in QA’s role is in conducting environmental audits. These audits evaluate not only compliance with GMP but also examine resource utilization, emissions, and waste generation. QA teams now collaborate with Environmental, Health, and Safety (EHS) departments to ensure compliance with environmental regulations and to monitor green performance indicators such as water footprint, energy intensity, and solvent recovery rates [7, 12].
Another critical aspect is sustainable documentation practices. Traditional paper-based quality systems are being replaced with digital platforms that reduce paper waste and support real-time data management. These platforms streamline data collection for sustainability KPIs (Key Performance Indicators), facilitate traceability, and improve audit readiness [27].
Furthermore, QA is increasingly involved in supporting EHS integration and green metrics tracking. QA systems are being redesigned to include metrics such as carbon intensity per batch, compliance with green chemistry principles, and percentage of renewable materials used. These parameters are now imgded into quality management systems to ensure that sustainability goals are monitored, validated, and continuously improved [21].
Table 3: Sustainability metrics for QA systems
| Sustainability metric | Description | Example for QA monitoring | Source |
| Carbon intensity | CO₂ emissions per unit batch or product | Carbon footprint audit | [24] |
| Water footprint | Total water used in production | Water reuse monitoring reports | |
| Solvent recovery rate | % solvents recovered and reused | Internal recovery validation in batch records | |
| Renewable raw material usage | % of materials sourced from renewable resources | Supplier audits and certification reports | |
| Hazardous Waste Reduction | % decrease in hazardous waste generation | Annual EHS report tracking |
Monitoring key sustainability indicators within QA systems ensures continuous environmental performance improvement. Table 3 provides an overview of relevant metrics used in pharmaceutical quality management [24].
The integration of sustainability into QA also enhances risk management. By evaluating environmental risks alongside product risks, QA contributes to a more holistic approach that anticipates and mitigates environmental harm while maintaining product integrity. Additionally, many companies are incorporating life cycle assessment (LCA) into QA protocols to evaluate the environmental impact of products from raw material sourcing to end-of-life disposal [16].
For example, LCA has been applied in evaluating the environmental trade-offs in API synthesis processes, where different chemical routes were compared to determine their impact on energy and waste generation. Additionally, packaging materials have been optimized using LCA to reduce plastic usage and improve biodegradability without compromising stability [35, 36].
QA’s evolution in the age of sustainability reflects a shift from reactive compliance to proactive stewardship, ensuring not only that medicines are safe and effective but also that their production respects ecological boundaries. This transformation positions QA as a key enabler of green innovation, operational transparency, and long-term environmental responsibility.
Digital QA and pharma 4.0 for sustainable manufacturing
The rise of Pharma 4.0 has ushered in a new era of digital transformation in the pharmaceutical industry, with significant implications for both quality assurance (QA) and sustainability. Digital Quality Management Systems (QMS) have become central to driving resource efficiency, regulatory compliance, and real-time decision-making, thereby aligning operational excellence with environmental responsibility [26].
Role of digital QMS in sustainable operations
The above fig. 1 explains how Digital QMS tools are integrated into pharmaceutical quality systems to improve sustainability. By enabling real-time monitoring, process optimization, and data-driven decision-making, these tools support reduced waste, improved visibility, and lower documentation burden.
Use of AI, IoT, and predictive analytics
Emerging technologies such as Artificial Intelligence (AI), Internet of Things (IoT), and predictive analytics enable real-time tracking of resource consumption and emissions. For example, AI algorithms can predict equipment failures and schedule maintenance, thereby reducing unscheduled downtimes and associated energy wastage. IoT sensors facilitate continuous environmental monitoring, tracking variables like temperature, pressure, and emissions with high accuracy to ensure compliance with green metrics [28]. Predictive tools also support lean inventory management and just-in-time production, minimizing excess stock and obsolescence [29].
Advanced concepts: real-time release testing, digital twins, and blockchain
Advanced Pharma 4.0 tools are now being integrated into QA frameworks to enhance efficiency and sustainability. Real-Time Release Testing (RTRT) enables product quality to be evaluated during manufacturing, reducing the need for large-scale end-product testing and minimizing batch rejections [25]. Digital Twins simulate entire production lines virtually, allowing companies to optimize energy use, identify bottlenecks, and run sustainability impact scenarios without disrupting real-time operations [28]. Additionally, blockchain-enabled supply chains offer immutable, transparent records of sourcing, production, and distribution, thereby supporting sustainability claims, traceability, and improved regulatory reporting [21].
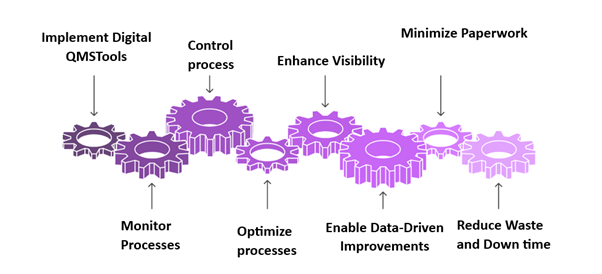
Fig. 1: Digital QMS process flow-illustrates how digital quality tools minimize paper use, optimize resource tracking, and enhance real-time sustainability monitoring (Source: Author’s own)
Enhancing data integrity in ESG and sustainability reporting
Digital QA systems ensure robust data integrity, which is vital for Environmental, Social, and Governance (ESG) reporting and regulatory compliance. With integrated analytics and automated data capture, digital systems reduce manual errors and offer verifiable audit trails. These capabilities are crucial for documenting adherence to Sustainable Development Goals (SDGs) and meeting investor and regulatory expectations around environmental performance [14].
QA across the product lifecycle: enabling sustainable practices
Quality assurance (QA) plays a pivotal role in imgding sustainability across the entire pharmaceutical product lifecycle from research and development (R and D) to distribution. In the early stages of drug discovery and development, QA ensures adherence to green chemistry principles by promoting the use of green solvents and eco-friendly synthesis routes [30, 31]. This not only minimizes hazardous waste generation but also reduces the overall environmental burden of pharmaceutical processes. During raw material sourcing, QA is responsible for verifying supplier practices to ensure ethical and sustainable procurement, thereby mitigating the risks associated with deforestation, biodiversity loss, and unethical labor practices. In active pharmaceutical ingredient (API) production and formulation, QA oversees the implementation of environmentally responsible manufacturing practices, such as reducing water and energy consumption, controlling emissions, and optimizing waste management systems [32, 33]. Furthermore, QA ensures the selection and validation of sustainable packaging materials that are biodegradable or recyclable, while also monitoring for harmful leachables and extractables, which contributes to reducing plastic pollution and aligning with responsible consumption goals. During distribution and storage, QA supports carbon-efficient logistics and energy-efficient cold chain systems to lower greenhouse gas emissions. At the end of the product lifecycle, QA facilitates take-back programs for expired or unused medications, enforces proper segregation of pharmaceutical and packaging waste, and supports safe disposal practices to prevent environmental contamination. Throughout all these stages, QA integrates life cycle assessment(LCA) tools to continuously evaluate the environmental impacts of pharmaceutical products and processes, including energy use, carbon footprint, and resource consumption. By imgding sustainability-focused checkpoints at every level, QA significantly contributes to greener, more resilient, and ethically aligned pharmaceutical manufacturing and distribution systems [34-37].
QA checkpoints from R and D to distribution with sustainability impact
In the pharmaceutical industry, integrating sustainability throughout the entire process from research and development (R and D) to distributionis essential. During the R and D phase, the selection of sustainable materials, such as biodegradable substances and eco-friendly packaging, plays a critical role in minimizing environmental impacts on landfills and ecosystems. The adoption of green chemistry principles is equally vital, as it focuses on reducing or eliminating hazardous substances and minimizing waste generation during drug development and manufacturing, thus enhancing sustainability while ensuring regulatory compliance [38]. Effective supply chain management is necessary to maintain sustainable practices throughout distribution, which involves sourcing raw materials from ethical and environmentally responsible suppliers [39]. Regulatory compliance is another key checkpoint, as the pharmaceutical industry faces increasing pressure to integrate sustainable practices due to environmental concerns and evolving legal requirements. Engaging stakeholders, including suppliers and customers, further enhances transparency and accountability, addressing the growing demand for responsible corporate behaviour [40]. Additionally, implementing monitoring systems to track sustainability metrics and adhering to Global Reporting Initiative (GRI) standards allow companies to disclose their environmental, social, and governance (ESG) impacts, improving accountability. Continuous improvement driven by researchers and academics plays a significant role in identifying opportunities to reduce environmental footprints and enhance sustainability throughout the pharmaceutical lifecycle. Lastly, integrating sustainability into pharmaceutical education is crucial for preparing future professionals to img green practices into their work, thereby fostering a culture of environmental responsibility and innovation within the industry [41, 42].
In the pharmaceutical industry, integrating sustainability throughout the entire process from research and development (R and D) to distribution is essential for minimizing environmental impact and promoting long-term viability. A key QA checkpoint begins with sustainable material development during the R and D phase, which includes selecting biodegradable materials and designing eco-friendly packaging to reduce the industry's burden on landfills and ecosystems. Another crucial aspect is the adoption of green chemistry principles, which aim to eliminate hazardous substances and reduce waste during drug development and manufacturing, thus enhancing both environmental sustainability and regulatory compliance. Effective supply chain management also plays a vital role in maintaining sustainability, as it ensures that raw materials are sourced from ethical and environmentally responsible suppliers, thereby reducing the environmental footprint of the distribution process. Compliance with environmental regulations is another essential checkpoint, as the industry is under increasing pressure to align its practices with evolving environmental policies, helping to mitigate ecological impacts and meet legal obligations. Engaging stakeholders such as suppliers and customers further enhances transparency and accountability in sustainability efforts, meeting growing demands for responsible corporate behaviour [43-45]. To support these initiatives, monitoring and reporting systems must be in place to track sustainability metrics, with adherence to frameworks like the Global Reporting Initiative (GRI) improving ESG accountability. Continuous improvement is driven by academic research and innovation, which helps identify strategies to lower the environmental footprint across the pharmaceutical lifecycle. Lastly, integrating sustainability into pharmaceutical education and training equips future professionals with the knowledge and mindset needed to implement green practices, fostering a culture of environmental responsibility within the industry [46-48].
Environmental governance and quality systems
Role of QA in implementing
The implementation of CI concepts in QA leads to the establishment of process performance indicators, which are crucial for evaluating overall organizational performance. This focus is aligned with contemporary requirements, such as those outlined in ICH Q10 and ISO 9004, which emphasize the importance of integrating quality management with cleaner production [49-51].
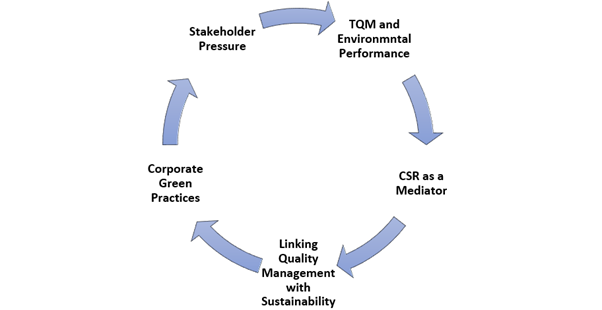
Fig. 2: TQM and sustainability cycle shows how total quality management drives corporate green practices through CSR, aligning quality systems with sustainability goals (Source: Author’s own)
The cycle presented in fig. 2 highlights the integrated relationship between total quality management (TQM), Corporate Social Responsibility (CSR), and sustainability goals. TQM frameworks strengthen process efficiency, while CSR initiatives serve as a bridge to align environmental performance with stakeholder expectations, thereby promoting Corporate Green Practices (CGP).
The study highlights that total quality management (TQM) practices can significantly enhance organizational capabilities to achieve green performance objectives, suggesting that effective quality management plays a crucial role in managing environmental risks by ensuring that processes are efficient and sustainable. Furthermore, Corporate Social Responsibility (CSR) acts as a partial mediator between TQM and Corporate Green Practices (CGP), implying that companies focusing on CSR can improve their environmental performance, which is essential for managing risks associated with sustainability. The research also emphasizes the importance of linking sustainable development objectives with quality management, helping organizations improve their overall sustainability performance and better address environmental risks [52]. The concept of CGP is discussed in terms of introducing new or improving existing products and processes to meet customer expectations while simultaneously enhancing environmental performance, aligning closely with the need for quality assurance (QA) in sustainability-linked data to ensure effectiveness and regulatory compliance. Additionally, the paper notes that businesses face increasing pressure from stakeholders to consider the environmental impact of their operations, necessitating robust environmental risk management strategies and QA processes to meet regulatory and sustainability goals [53-55].
Building a sustainable quality culture
imgding sustainability into quality assurance (QA) practices is becoming increasingly important, with a focus on integrating sustainability perspectives into Quality Management (QM) systems. Organizations are encouraged to review and update their standard operating procedures (SOPs), training programs, and audit protocols to reflect sustainability goals, ensuring that QA efforts align with broader sustainable development objectives. A key shift highlighted is the move from focusing solely on customer satisfaction to achieving societal satisfaction, which requires QA teams to adopt a sustainability-first mindset in their operations and decision-making processes [55, 56]. To support this shift, upskilling staff is essential, particularly in areas such as understanding climate impact, applying green metrics, and implementing sustainable operations. The development of additional quality management models aimed at societal satisfaction further emphasizes the need for sustainability-related competencies. Additionally, cross-functional collaboration among departments such as QA, Operations, Environmental Health and Safety (EHS), and Regulatory Affairs is crucial. Redefining the quality function to better meet stakeholder expectations facilitates the development of innovative, sustainable solutions that address the rapidly evolving demands of society [57, 58].
Barriers and strategic enablers
For quality assurance (QA) teams in the pharmaceutical industry, understanding sustainability metrics that encompass environmental, social, and economic aspects is essential for effectively evaluating the sustainability of products and processes. Structured frameworks such as scorecards and sustainability indices can support this understanding by enabling the assessment and comparison of different product-service systems. Additionally, training QA teams in life cycle assessment (LCA) and Life Cycle Costing (LCC) methodologies is crucial, as these tools offer valuable insights into the environmental impacts of products throughout their entire life cycle, allowing for more informed decision-making [59]. Cross-functional collaboration among QA, Operations, Environmental Health and Safety (EHS), and Regulatory teams is equally important for imgding sustainability practices organization-wide; for example, integrating a 'green strategy' into Corporate Social Responsibility (CSR) programs can strengthen both sales and corporate commitment to sustainability. Upskilling staff to understand climate impacts, green metrics, and sustainable manufacturing practices is vital for empowering QA teams to actively contribute to sustainability initiatives. Moreover, adopting sustainable practices not only fulfills social responsibilities but also enhances a company's reputation as a 'green' organization, leading to greater customer satisfaction. However, several challenges hinder the transition to greener practices, including a lack of research into the drivers and barriers for different pharmaceutical sectors, such as innovative, generic, and biopharma companies. Regulatory challenges, such as stringent quality requirements and lengthy approval processes, can discourage green modifications in drug development due to compliance risks. Furthermore, the absence of standardized green QA frameworks creates inconsistencies in the implementation and measurement of sustainability initiatives, complicating efforts to align total quality management (TQM) and good manufacturing practices (GMP) with sustainability goals [59]. Measurement issues related to sustainability Key Performance Indicators (KPIs) within QA systems further impede the evaluation of environmental performance and the effectiveness of green practices, highlighting the need for more empirical research. Finally, cultural and structural resistance within legacy systems poses a significant barrier, as traditional mindsets often resist the changes necessary for integrating sustainability. Overcoming these challenges requires fostering a culture of employee empowerment and involvement to facilitate the successful adoption of sustainable practices in pharmaceutical quality systems [60].
Strategic enablers
The integration of total quality management (TQM) into manufacturing processes provides a comprehensive framework that supports the transition to environmentally sustainable practices. By emphasizing process efficiency and waste reduction, TQM enhances the adoption of good manufacturing practices (GMP) initiatives, positioning organizations for greater success. Employee engagement and empowerment are also critical, as encouraging staff to identify areas for improvement fosters innovative solutions to sustainability challenges. TQM promotes a culture where employees are motivated to contribute ideas for reducing waste and advancing eco-friendly practices, thereby strengthening green initiatives. Strong leadership and strategic planning are equally important for aligning quality management with sustainability objectives [61]. Assimilating environmental sustainability into quality management frameworks can be particularly transformative for small and medium-sized enterprises (SMEs), facilitating the wider adoption of GMP practices. Furthermore, digital transformation plays a pivotal role by improving data collection and analysis, enabling better decision-making in process assessments. The use of digital tools allows for more efficient monitoring of chemical processes and their environmental impacts, supporting the implementation of green chemistry practices. Regulatory incentives and harmonization across regions are also crucial, as streamlined regulations and incentives for using fewer toxic materials or environmentally friendly processes encourage companies to innovate and adopt sustainable methods. Finally, cross-industry collaboration and benchmarking serve as powerful drivers for progress, enabling companies to share knowledge, learn from best practices, and accelerate the development and implementation of greener technologies and processes [62-64].
The paper emphasizes that these enablers are essential for the successful application of the intensification factor (IF) in assessing and improving chemical processes. The IF serves as a decision-making tool that incorporates various qualitative and quantitative factors, aiding in the comparison of different chemical strategies and their sustainability impacts [65, 66].
CONCLUSION
In the face of escalating environmental concerns and evolving regulatory expectations, the pharmaceutical industry must embrace sustainability not as an option but as a core operational imperative. This review has highlighted the pivotal transformation of quality assurance (QA) from a compliance-centric function to a strategic driver of sustainable manufacturing. By integrating green chemistry principles, digital quality systems, life cycle assessments, and sustainability metrics, QA now plays a central role in reducing the industry's ecological footprint while maintaining product integrity and regulatory compliance.
Digital tools under the Pharma 4.0 paradigm, combined with cross-functional collaboration, employee empowerment, and stakeholder engagement, have further enhanced QA's capacity to drive innovation in sustainable practices. Nevertheless, achieving this vision requires overcoming persistent barriers such as regulatory rigidity, lack of standardized green QA frameworks, and cultural resistance. Strategic enablers, ranging from TQM integration and leadership commitment to policy incentives and digital transformation, must be leveraged to img sustainability across the pharmaceutical product lifecycle.
Ultimately, aligning QA with environmental stewardship not only strengthens corporate social responsibility but also enhances long-term resilience, operational efficiency, and public trust. The path forward lies in imgding sustainability into the very DNA of pharmaceutical quality systems, ensuring that future medicines are not only safe and effective but also responsibly manufactured for the health of both people and the planet.
ACKNOWLEDGEMENT
We are grateful to Nitte (Deemed to be University) and the Department of Pharmaceutics, NGSM Institute of Pharmaceutical Sciences – Mangalore, for enabling us to be a part of this course.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
Vineeth Raj K: Literature review, Data curation, Writing-original draft, and Evaluation; Raveesh Gopal Hegde: Literature review, Data curation, Writing-original draft, and Evaluation; Anoop Narayanan V: Review and editing, Supervision, Evaluation, Visualization.
CONFLICTS OF INTERESTS
The authors declare no conflict of interest.
REFERENCES
Desai M, Njoku A, Nimo Sefah L. Comparing environmental policies to reduce pharmaceutical pollution and address disparities. Int J Environ Res Public Health. 2022 Jul 7;19(14):8292. doi: 10.3390/ijerph19148292, PMID 35886145, PMCID PMC9325029.
Bade C, Olsacher A, Boehme P, Truebel H, Burger L, Fehring L. Sustainability in the pharmaceutical industry an assessment of sustainability maturity and effects of sustainability measure implementation on supply chain security. Corp Soc Responsibility Env. 2024;31(1):224-42. doi: 10.1002/csr.2564.
Milanesi M, Runfola A, Guercini S. Pharmaceutical industry riding the wave of sustainability: review and opportunities for future research. J Clean Prod. 2020 Mar 19;261:121204. doi: 10.1016/j.jclepro.2020.121204.
Lee BX, Kjaerulf F, Turner S, Cohen L, Donnelly PD, Muggah R. Transforming our world: implementing the 2030 agenda through sustainable development goal indicators. J Public Health Policy. 2016 Sep;37Suppl 1:13-31. doi: 10.1057/s41271-016-0002-7, PMID 27638240.
Riikonen S, Timonen J, Sikanen T. Environmental considerations along the life cycle of pharmaceuticals: interview study on views regarding environmental challenges, concerns, strategies and prospects within the pharmaceutical industry. Eur J Pharm Sci. 2024 May 1;196:106743. doi: 10.1016/j.ejps.2024.106743, PMID 38460610.
Heberer T, Ternes TA. Pharmaceuticals and the environment. J Environ Manag. 2020 Nov 1;270:110702. doi: 10.1016/j.jenvman.2020.110702.
Nasrullah M, Khan SA, Hashmi MZ, Iqbal J. Green manufacturing, green chemistry and environmental sustainability: a review. Curr Res Environ Sustain. 2019;1:100003.
Karina Witte, Michael Muller. Sustainable pharmacy: a guiding principle. Sustainable Chemistry and Pharmacy. 2025 Apr;44:101897. doi: 10.1016/j.scp.2024.101897.
Belkhir L, Elmeligi A. Carbon footprint of the global pharmaceutical industry and relative impact of its major players. J Cleaner Prod. 2019 Mar 20;214:185-94. doi: 10.1016/j.jclepro.2018.11.204.
Olawade DB, Popoola TT, Egbon E, David Olawade AC. Sustainable healthcare practices: pathways to a carbon-neutral future for the medical industry. Sustain Futures. 2025 Jun;9:100783. doi: 10.1016/j.sftr.2025.100783.
Di Russo M, Zjalic D, Lombardi GS, Perilli A, Congedo G, Daugbjerg S. Impact of the 50 biggest pharma companies: a review of environmental report aspiring to NetZero. Eur J Public Health. 2023 Oct 1;33Suppl 2:ckad160.1182. doi: 10.1093/eurpub/ckad160.1182.
El Haouat Z, Essalih S, Bennouna F, Amegouz D. Development of a global framework for an integrated life cycle assessment (LCA) model in quality safety and environmental (QSE) management systems: improving environmental, social and economic sustainability performance. Sustainability. 2025 Apr 14;17(8):3521. doi: 10.3390/su17083521.
Sonu GM, Rani GM, Pathania D, Abhimanyu S, Umapathi R, Rustagi S. Agro-waste to sustainable energy: a green strategy of converting agricultural waste to nano-enabled energy applications. Sci Total Environ. 2023 Jun 1;875:162667. doi: 10.1016/j.scitotenv.2023.162667, PMID 36894105.
Saikiran P, Pawan Kumar T, Arya S, Tijare D, Loharkar S, Bajad G. Advances in pharmaceutical oral solid dosage forms. In: Jain K, Yadav AK, editors. In advances in pharmaceutical product development. Singapore: Springer Nature; 2025 Mar 19. p. 111-42. doi: 10.1007/978-981-97-9230-6_5.
Mustoe CL, Turner AJ, Urwin SJ, Houson I, Feilden H, Markl D. Quality by digital design to accelerate sustainable medicines development. Int J Pharm. 2025 Aug 20;681:125625. doi: 10.1016/j.ijpharm.2025.125625, PMID 40287074.
Agrawal M, Bansal A, Khandelwal VK, Bansal N. Sustainable pharma: the need, current status and mission for the future. Scr Med. 2024;55(4):489-99. doi: 10.5937/scriptamed55-51612.
Belal MM, Shukla V, Ahmad S, Balasubramanian S. Green Pharma supply chain: a review of existing practices and future directions. Manag Environ Qual. 2025;36(1):72-106. doi: 10.1108/MEQ-08-2023-0249.
Chaudhary M, Choudhary P, Tripathi A, Pandey VK, Sharma R, Singh S. Pharmaceutical orientation and applications of silver/zinc oxide nanoparticles developed from various fruit peel extracts: an emerging sustainable approach. Discov Sustain. 2025 Jan 6;6(1):7. doi: 10.1007/s43621-024-00728-y.
Chen Z, Lian JZ, Zhu H, Zhang J, Zhang Y, Xiang X. Application of life cycle assessment in the pharmaceutical industry: a critical review. J Cleaner Prod. 2024 May 11;459:142550. doi: 10.1016/j.jclepro.2024.142550.
Sinha M, Kumari P. Sustainability transformation through green supply chain in pharma industry. Int J Supply Oper Manag. 2021;8(2):123-32.
Diaz De Junguitu A, Allur E. The adoption of environmental management systems based on ISO 14001, EMAS, and alternative models for SMEs: a qualitative empirical study. Sustainability. 2019 Dec 9;11(24):7015. doi: 10.3390/su11247015.
Jindal R, Singh D. Integration of green chemistry principles in pharmaceutical production. Int J Green Chem. 2023;12(1):45-58.
Sharma V, Kumar A. Evaluating sustainability metrics for pharmaceutical quality systems. J Environ Chem Eng. 2022;10(2):124-34.
Priyadarshini D. Sustainable innovation in pharmaceutical manufacturing: future of medicine production. Curr Opin Green Sustain Chem. 2025;42:100872.
Pandey A, Joshi H. Optimizing quality standards in Pharma 4.0 by implementation of QMS. Int J Pharm Sci Rev Res. 2023;81(1):1-6.
Verma R, Gupta A. Digital transformation and Industry 4.0 in pharma manufacturing. J Pharm Technol. 2022;38(3):160-6.
Hasan M, Ali S. A systematic review of Industry 4.0 technologies in pharmaceutical manufacturing. Sustain Ind. 2023;2(1):1-12.
Kodumuru R, Sarkar S, Parepally V, Chandarana J. Artificial intelligence and internet of things integration in pharmaceutical manufacturing: a smart synergy. Pharmaceutics. 2025 Feb 22;17(3):290. doi: 10.3390/pharmaceutics17030290, PMID 40142954.
Kabir M, Rana MR, Debnath A. The role of quality assurance in accelerating pharmaceutical research and development: strategies for ensuring regulatory compliance and product integrity. J Angiother. 2024 Dec 8;8(12):1. doi: 10.25163/angiotherapy.81210102.
Ramanathan N. imgding sustainability concerns into quality assurance. Total Qual Manag Bus Excell. 2025 Feb 17;36(3-4):249-63. doi: 10.1080/14783363.2020.1858712.
Milisavljevic Syed J, Khan M, Xia H, Li J, Salonitis K. Charting the course: standardization of quality assurance in digital twin applications across product lifecycle. Procedia CIRP. 2024 Jan 1;130:718-23. doi: 10.1016/j.procir.2024.10.154.
Faieq HT, Cek K. Enhancing kurdistans manufacturing companies sustainable waste management: a norm activation approach to green accounting, CSR, and environmental auditing oversight. Heliyon. 2024 Jun 30;10(12):e32725. doi: 10.1016/j.heliyon.2024.e32725, PMID 38975142.
Bhadoriya A, Patil B, Vinchurkar K, Mane S, Parambath A. Materials sustainability in the pharmaceutical industry. Sustainability & Circularity NOW. 2024;1:a24604207. doi: 10.1055/a-2460-4207.
Belal MM, Shukla V, Ahmad S, Balasubramanian S. Green pharma supply chain: a review of existing practices and future directions. Manag Environ Qual. 2025 Jan 29;36(1):72-106. doi: 10.1108/MEQ-08-2023-0249.
Chen Z, Lian JZ, Zhu H, Zhang J, Zhang Y, Xiang X. Application of life cycle assessment in the pharmaceutical industry: a critical review. J Clean Prod. 2024 May 11;459:142550. doi: 10.1016/j.jclepro.2024.142550.
Zhang B, Mohammad J. Sustainability of perishable food cold chain logistics: a systematic literature review. Sage Open. 2024 Sep;14(3):21582440241280455. doi: 10.1177/21582440241280455.
Ashiwaju BI, Orikpete OF, Fawole AA, Alade EY, Odogwu C. A step toward sustainability: a review of biodegradable packaging in the pharmaceutical industry. Matrix Sci Pharm. 2023;7(3):73-84. doi: 10.4103/mtsp.mtsp_22_23.
Awaysheh A, Klassen RD. The impact of supply chain structure on the use of supplier socially responsible practices. Int J Oper Prod Manag. 2010 Nov 16;30(12):1246-68. doi: 10.1108/01443571011094253.
Vian T, Kohler JC, Forte G, Dimancesco D. Promoting transparency accountability and access through a multi-stakeholder initiative: lessons from the medicines transparency alliance. J Pharm Policy Pract. 2017 Dec;10(1):18. doi: 10.1186/s40545-017-0106-x, PMID 28588896.
Hassan AS, Jaaron AA. Total quality management for enhancing organizational performance: the mediating role of green manufacturing practices. J Cleaner Prod. 2021 Jul 25;308:127366. doi: 10.1016/j.jclepro.2021.127366.
Bade C, Olsacher A, Boehme P, Truebel H, Burger L, Fehring L. Sustainability in the pharmaceutical industry an assessment of sustainability maturity and effects of sustainability measure implementation on supply chain security. Corp Soc Responsibility Env. 2024 Jan;31(1):224-42. doi: 10.1002/csr.2564.
Gul RF, Jamil K, Mustafa S, Jaffri NR, Anwar A. Mitigating the environmental concerns through total quality management and green manufacturing practices. Environ Sci Pollut Res Int. 2024 Jun;31(27):39285-302. doi: 10.1007/s11356-024-33826-5, PMID 38814557.
Hassan AS, Jaaron AA. Total quality management for enhancing organizational performance: the mediating role of green manufacturing practices. J Clean Prod. 2021 Jul 25;308:127366. doi: 10.1016/j.jclepro.2021.127366.
Ogbuagu OO, Mbata AO, Balogun OD, Oladapo O, Ojo OO, Muonde MU. Sustainable pharmaceutical supply chains: green chemistry approaches to drug production and distribution. IRE J. 2024 Oct;8(4):761-7.
Daughton CG. Cradle to cradle stewardship of drugs for minimizing their environmental disposition while promoting human health. I. rationale for and avenues toward a green pharmacy. Environ Health Perspect. 2003 May;111(5):757-74. doi: 10.1289/ehp.5947, PMID 12727606.
Kabir M, Rana MR, Debnath A. The role of quality assurance in accelerating pharmaceutical research and development: strategies for ensuring regulatory compliance and product integrity. J Angiotherapy. 2024 Dec 8;8(12):1. doi: 10.25163/angiotherapy.81210102.
Helwig K, Niemi L, Stenuick JY, Alejandre JC, Pfleger S, Roberts J. Broadening the perspective on reducing pharmaceutical residues in the environment. Environ Toxicol Chem. 2024 Mar 1;43(3):653-63. doi: 10.1002/etc.5563, PMID 36647735.
Karapetrovic S, Willborn W. Integration of quality and environmental management systems. TQM Mag. 1998 Jun 1;10(3):204-13. doi: 10.1108/09544789810214800.
Seymour EJ, Ridley AM, Noonan J. Assessing the role of a four-stage approach for improving the compatibility of environmental management systems and quality assurance. Aust J Exp Agric. 2007 Feb 12;47(3):333-45. doi: 10.1071/EA06026.
Battersby S. Quality management systems: environmental management systems as examples. In: clay’s handbook of environmental health. Routledge; 2022 Aug 16. p. 242-76.
Abbas J. Impact of total quality management on corporate green performance through the mediating role of corporate social responsibility. J Clean Prod. 2020 Jan 1;242:118458. doi: 10.1016/j.jclepro.2019.118458.
Giram PS, Gaikwad VV, Thonte SS, Rajurkar MR, Gholve SB, Bhusnure OG. Environmental protection by implementation of green purchasing, green productivity, green marketing and green quality management systems. Environ Manag. 2015 Aug 4;4(10):2005-28.
Gujral SK. Green marketing sustainable development. Int J Manag Soc Sci. 2016;4(3):66-70.
Bastas A. Sustainable manufacturing technologies: a systematic review of latest trends and themes. Sustainability. 2021 Apr 12;13(8):4271. doi: 10.3390/su13084271.
Deleryd M, Fundin A. Towards societal satisfaction in a fifth generation of quality the sustainability model. Total Qual Manag Bus Excell. 2025 Feb 17;36(3-4):292-308. doi: 10.1080/14783363.2020.1864214.
Chen H, Akparep J, Sulemana I, Osei A. Advancing green innovations in pharmaceutical firms towards societal development: nurturing customers health and building loyalty. Environ Dev Sustain. 2024 Nov 15:1-26. doi: 10.1007/s10668-024-05690-3.
Nahas N, Chandrasekar KS. Total quality management in pharmaceutical industry: with respect to green innovation. J Gujarat Res Soc. 2019;21(8):242-8.
Aboueid S, Beyene M, Nur T. Barriers and enablers to implementing environmentally sustainable practices in healthcare: a scoping review and proposed roadmap. Healthc Manage Forum. 2023 Nov;36(6):405-13. doi: 10.1177/08404704231183601, PMID 37357691.
Faisal M. Research analysis on barriers to green supply chain management in pharmaceutical industries. Review Pub Administration Manag. 2015;3(1):1-5. doi: 10.4172/2315-7844.1000176.
Zaman SI, Ali MR, Khan SA. Exploring interrelationships among barriers and enablers of green procurement for a sustainable supply chain. Int J Procurement Manag. 2024;19(2):226-52. doi: 10.1504/IJPM.2024.136051.
Chaturvedi U, Sharma M, Dangayach GS, Sarkar P. Evolution and adoption of sustainable practices in the pharmaceutical industry: an overview with an Indian perspective. J Clean Prod. 2017 Dec 1;168:1358-69. doi: 10.1016/j.jclepro.2017.08.184.
Belal MM, Shukla V, Ahmad S, Balasubramanian S. Green Pharma supply chain: a review of existing practices and future directions. Manag Environ Qual. 2025 Jan 29;36(1):72-106. doi: 10.1108/MEQ-08-2023-0249.
TS N, Bharath S, PN, BR M. Harmonizing quality and sustainability: a comprehensive analysis of total quality management and green manufacturing practices in small and medium-sized enterprises. Int J Lean Six Sigma. 2025 Feb 6. doi: 10.1108/IJLSS-10-2024-0236.
Fernandez Rivas DF, Cintas P. On an intensification factor for green chemistry and engineering: the value of an operationally simple decision-making tool in process assessment. Sustain Chem Pharm. 2022 Jun 1;27:100651. doi: 10.1016/j.scp.2022.100651.
Deleryd M, Fundin A. Towards societal satisfaction in a fifth generation of quality the sustainability model. Total Qual Manag Bus Excell. 2025 Feb 17;36(3-4):292-308. doi: 10.1080/14783363.2020.1864214.
Laddha PR, Sitaphale GR, Charhate KB, Tathe PR. Green chemistry in pharmaceutical synthesis: sustainable strategies for drug production. Intr J Adv Res Sci Commun Technol. 2024;4(2):323-30.
Sontakke GM, Sakhare SS, Chavan RD, Dhakne GS, Hamand VG. Quality control and quality assurance in pharmaceutical industry. Intr J Adv Res Sci Commun Technol. 2023;3(2):501-10.