
Int J Pharm Pharm Sci, Vol 17, Issue 10, 33-44Original Article
“TARGETING MULTI-MICROBIAL INFECTION IN CHRONIC WOUNDS THROUGH TRIPLE ANTIBIOTIC NANOEMULGEL FORMULATION”
NIPA THACKER1, RICHA DAYARAMANI2*, SUNNY RATHEE2
1Gujarat Technological University, Chandkheda, Ahmedabad, Gujarat-382424, India. 2Centre of Excellence in Medical Devices, National Institute of Pharmaceutical Education and Research-Ahmedabad (NIPER-A), Opposite Air Force Station, Palaj, Gandhinagar-382355, Gujarat, India
*Corresponding author: Richa Dayaramani; *Email: richadayaramani1976@gmail.com
Received: 08 Jul 2025, Revised and Accepted: 25 Aug 2025
ABSTRACT
Objective: Chronic diabetic wounds are challenging to treat due to multi-microbial infections. This study developed an innovative triple-antibiotic nanoemulgel formulation (TANF) that integrates metronidazole, norfloxacin, and mupirocin, targeting anaerobic bacteria, Gram-negative bacteria, and Gram-positive bacteria, respectively. The objective was to create a wide-spectrum, effective topical therapy with superior drug delivery characteristics.
Methods: Six nanoemulgel batches were prepared using high-speed homogenization, with 0.5% Hydroxypropyl Methylcellulose (HPMC) E15 as the gelling agent in batch F1. The formulation incorporated tea tree oil and coconut oil as penetration enhancers. Comprehensive physicochemical characterization included drug content, pH, spreadability, skin permeability, and in vitro drug release studies. Drug release kinetics was analyzed, and stability investigations were initiated.
Results: Batch F1 demonstrated optimal performance, achieving drug release of 90.12% Metronidazole, 89.56% Norfloxacin, and 90.79% Mupirocin within 24 h. The formulation maintained pH levels between 6-6.5, over 95% drug content, and excellent spreadability. Higuchi kinetics governed the drug release, and the inclusion of penetration enhancers significantly improved skin permeability. The nanoemulgel exhibited reduced greasiness and a dual control release mechanism, addressing limitations in traditional topical formulations.
Conclusion: The developed TANF demonstrated strong, broad-spectrum antimicrobial activity and enhanced drug delivery, making it a promising topical therapy for chronic diabetic wounds. Its optimized formulation ensures effective microbial coverage and improved patient compliance. Ongoing stability studies will confirm its long-term safety and efficacy.
Keywords: Nanoemulgel, Triple-antibiotic, Metronidazole, Norfloxacin, Mupirocin, Higuchi kinetics, Diabetic wound
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijpps.2025v17i10.55958 Journal homepage: https://innovareacademics.in/journals/index.php/ijpps
INTRODUCTION
A nanoemulgel is typically a biphasic system, where one immiscible liquid is finely dispersed into another, stabilized by emulsifying agents, and incorporated into a gel matrix, providing an effective medium for the delivery of both hydrophilic and hydrophobic drugs. Nanoemulgels provide superior characteristics, including thixotropic, greaseless nature, ease of application, emollience, non-staining properties, and skin friendliness, making them highly favourable for topical drug delivery [1, 2]. The dual-control release mechanism of nanoemulgels combines the stability of emulsions and the consistency of gels while creating a stable and aesthetic formulation that enhances drug release and penetration. This property is particularly advantageous for hydrophobic drugs, which are otherwise challenging to integrate directly into gel-based formulations. Additionally, the gel network offers superior drug-loading capacity compared to traditional carriers such as liposomes or niosomes [3-5]. The ability to incorporate hydrophobic drugs into the oil phase improves solubility and stability, leading to better drug release profiles. The system’s enhanced stability, cost-effectiveness, and simpler production requirements, avoiding intensive sonication and expensive materials, further underscore its utility. Moreover, the incorporation of permeation enhancers such as menthol or isopropyl myristate ensures increased skin permeability, facilitating effective drug delivery [6-8]. Key ingredients in TANFs include aqueous materials, oils, emulsifiers, gelling agents, co-surfactants, permeation enhancers, and preservatives. Aqueous agents such as water or alcohols are chosen based on the solubility of the drug, while essential oils like tea tree oil not only solubilize hydrophobic drugs but also act as penetration enhancers. Emulsifiers, such as Tween 80 and Span 80, stabilize the inherently unstable emulsions, while gelling agents like HPMC E15/K15 provide the necessary consistency. The polymer matrix of HPMC E15 was prepared. Co-surfactants and preservatives further enhance emulsification and protect the formulation against microbial growth, respectively [9, 10]. Metronidazole is available as a fixed-dose combination with both mupirocin and norfloxacin on the market. This study focuses on the formulation and characterization of a triple-antibiotic nanoemulgel aimed at improving the topical delivery of antibiotics. Metronidazole is effective against anaerobic bacteria, norfloxacin against g-negative bacteria, and mupirocin against g-positive bacteria. The formulation utilises the complementary spectrum of activity to address the complex microbial environment of chronic diabetic wounds delivered through a TANF, thereby leveraging the unique properties of nanoemulgels and the synergistic action of the antibiotics to overcome limitations of poor stability, low bioavailability, and inadequate skin penetration. The incorporation of tea tree oil and coconut oil further enhances skin permeability and therapeutic efficacy, paving the way for more effective and patient-friendly therapeutic solutions [11-13]. This study aimed to formulate and evaluate a novel TANF incorporating Metronidazole, Norfloxacin, and Mupirocin for the effective management of chronic diabetic wounds. The goal was to achieve broad-spectrum antimicrobial activity by targeting anaerobic, Gram-negative, and Gram-positive bacteria, while enhancing drug delivery, skin permeability, and patient compliance through a stable, non-greasy, and easy-to-apply topical system.
MATERIALS AND METHODS
Material required
Metronidazole, Mupirocin, Norfloxacin, were obtained as gift samples from Stallion Pharma Ltd, Ahmedabad, and Carpobol 934, HPMC E15, Polysorbate T 80, Propylene Glycol, Glycerine, EDTA, Citric Acid, Phenoxyethanol from SD Fine, and Coconut oil, Tea tree oil, Aloe vera Gel, were purchased from a local ayruvedic pharmacy. Distilled water was used for all experiments.
Equipment used
A high-speed homogenisation technique was employed for the preparation of the nanoemulgel. The instruments used are FTIR, HPLC with UV detector, pH meter, Brookfield Viscometer (DV-II+pro) with spindle S64, Zetasizer Nano ZS instrument (Malvern Instruments, USA), and Franz diffusion cell apparatus.
Preformulation studies
Drug excipient compatibility
The FTIR spectra of Metronidazole, Mupirocin, and Norfloxacin, in combination with excipients like Polysorbate T 80, Propylene Glycol, Coconut Oil, Tea Tree Oil, Glycerine, EDTA, Citric Acid, Aloe Vera Gel, and Phenoxyethanol, provide critical insights into drug-excipient compatibility. Compatibility studies are essential for ensuring the stability, efficacy, and safety of pharmaceutical formulations [15-17].
The spectral analysis displayed characteristic peaks of the individual drugs, representing their functional groups. Upon blending with excipients, these peaks were carefully examined to identify any shifts, reductions in intensity, or the emergence of new peaks. We looked for changes that could signify interactions, including chemical incompatibilities or physical alterations in the formulation [18-20].
In the provided spectra, the characteristic functional group regions, such as C=O stretching, O-H bending, and C-H vibrations, were monitored. The absence of significant changes in the FTIR peaks of the drugs after combining them with the excipients suggests that the drugs remain stable in the presence of these excipients. This observation is crucial for ensuring that the therapeutic activity of the drugs is not compromised. Polymers such as HPMC K15 and HPMC E15 were evaluated for their gelling efficiency, and HPMC E15 was selected as the optimal gelling agent due to its ability to provide suitable viscosity for effective drug release.
Excipients like Polysorbate T 80 and Propylene Glycol serve as solubilizing agents, while Aloe Vera Gel is a humectant and soothing agent, and Tea Tree Oil acts as bio bioenhancer. The resultsconfirm that these excipients can be safely used with metronidazole, mupirocin, and norfloxacin, forming a stable formulation suitable for therapeutic applications (fig. 1).
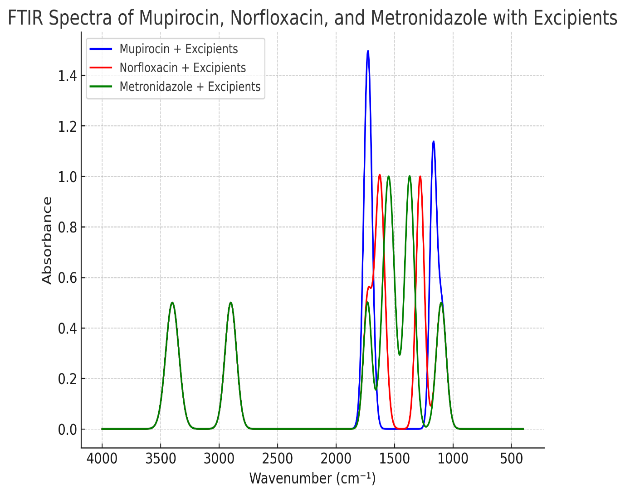
Fig. 1: Drug excipient compatibility through FTIR spectroscopy
Preparation of TANF
Step 1: Preparation of gel base
The gel base in the formulations is prepared by dispersing HPMC E15 in a small amount of cold water to form a viscous solution which is stored for 24 h in refrigerator [21, 22].
Step 2: Preparation of emulsion
The oil phase was made by dissolving PEG 150 stearate in coconut oil and tea tree oil. Drugs were dissolved in a mixture of propylene glycol and ethanol and then added to the oil phase. The aqueous phase was made by dissolving tween 80, EDTA and citric acid in water and mixing it with the mixture of aloe-vera gel and glycerin. To this aqueous phase phenoxy ethanol was added. The oily phase was added in small portions to the aqueous phase along with continuous homogenizing for 2 h at 4000 rpm [23, 24].
Step 3: Preparation of nanoemulgel
The nanoemulsion was combined with the gel base in a 1:1 ratio with constant stirring at 350 rpm for 1 h. After this, the contents of gel were homogenised at 2500-4000 RPM for 30 min. The gel was allowed to stabilize for 12 h. Check pH, viscosity and clarity [25-27].
Characterization of tanf
Physical examination
Visual inspection of the prepared TANF batches exhibited a homogenous uniform white colour with smooth texture and no grittiness. The formulations did not exhibit any phase separation after 48 h at room temperature [28-30].
pH
Using digital pH meter, one g of TANF was dispersed in 10 ml of distilled water and measurements were performed in triplicate [31-34]. The pH of all batches ranged between 6.0-6.5, which is appropriate for topical application.
Spreadability test
Spread ability was assessed by placing 1 g of each TANF between two glass slides for 5 min. The diameter of the resulting spread was measured in centimetres. An inverse relationship was observed between viscosity and spreadability. Results indicated that increasing polymer concentration led to a corresponding decrease in spread ability.
Viscosity
Samples (10 ml) were equilibrated at 25±1 °C for 30 min prior to analysis. The spindle was carefully positioned in the centre of the nanoemulgel to avoid contact with the container surfaces and rotated at 60 rpm, with readings recorded after 10 min. Results demonstrated a direct correlation between polymer concentration and viscosity.
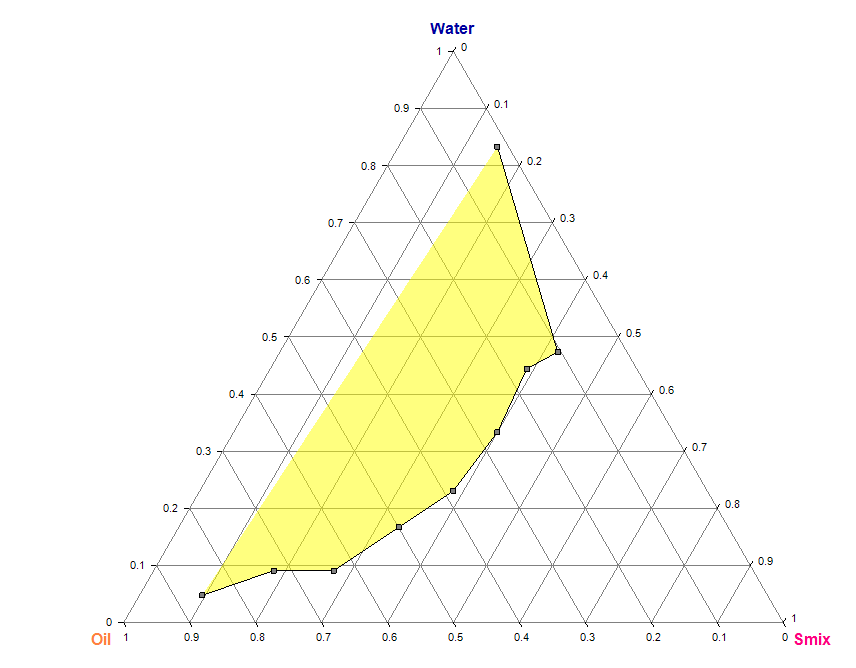
Pseudo ternary diagram for nanoemulgel preparation
The phase (water) titration method was used to construct pseudo-tertiary phase diagrams and determine the concentration range of components for the nanoemulgel region. After studying the solubility of drugs in different oils, surfactants, and co-surfactants, the components that showed the highest solubility were selected for constructing the pseudo-ternary phase diagrams. Four phase diagrams were constructed using different weight ratios of the surfactant/co-surfactant mixture 1:1, 2:1, 1:2, and 1:4. The oil phase was then combined with the surfactant mixture in varying weight ratios of 0.9:0.1, 0.8:0.2, 0.7:0.3, 0.6:0.4, 0.5:0.5, 0.4:0.6, 0.3:0.7, 0.2:0.8, and 0.1:0.9. These combinations were diluted dropwise with aqueous phase while being moderately agitated using a mixer at room temperature.
The samples were gradually titrated with water until they exhibited turbidity or phase separation. The endpoint of aqueous phase was determined when the sample became a turbid, biphasic (two-phase) dispersed medium. Samples that remained transparent and single-phase dispersed systems were identified as microemulsions. The results obtained from the water titration method were then entered into the ProSim software to construct pseudo-ternary phase diagrams (fig. 2). Table 1 represents the composition of topical nanoemulgel formulations (F1–F6) using different concentrations of HPMC E15 and HPMC K15 as gelling agents.
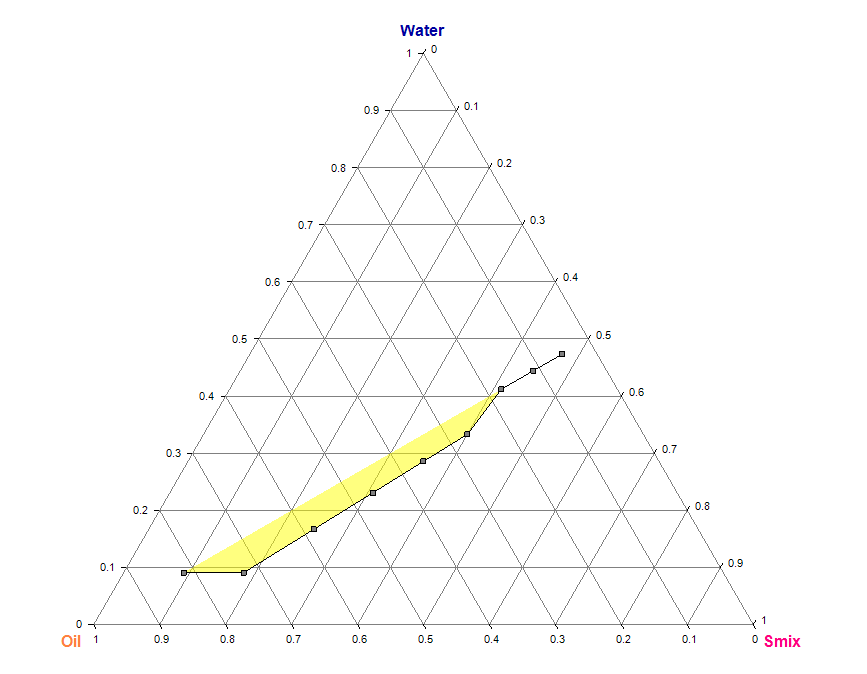
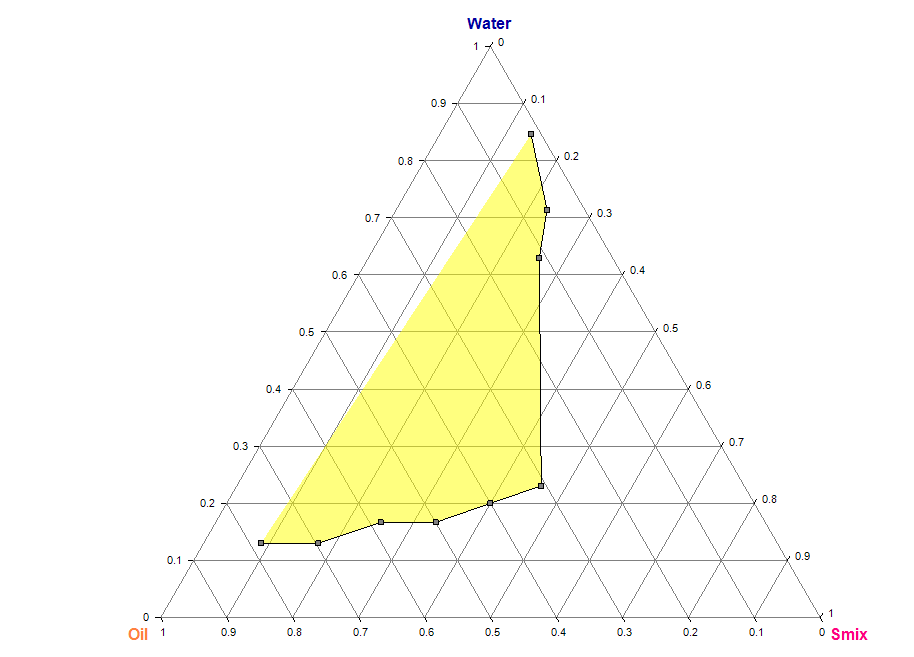
Pseudo-ternary phase diagram for (1:1) Smix ratio Pseudo-ternary phase diagram for (1:2) Smix ratio
Pseudo-ternary phase diagram for (1:3) Smix ratio Pseudo-ternary phase diagram for (1:4) Smix ratio
Fig. 2: Pseudo-ternary phase diagrams of the TANF system prepared using tea tree oil and coconut oil as the oil phase, and Smix ratios of 1:1, 1:2, 1:3, and 1:4 (Tween 80:Propylene Glycol). Each diagram indicates the nanoemulsion region based on clear, isotropic, and single-phase zones observed during titration
Table 1: Formulation of topical nanoemulgel (%w/w)
| Ingredient | F1 | F2 | F3 | F4 | F5 | F6 |
| Drug (200 mg in 5 ml of ethanol) | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 |
| HPMC E15 (% w/w) | 0.5 | 1.0 | 1.5 | - | - | - |
| HPMC K15 (% w/w) | - | - | - | 0.5 | 1.0 | 1.5 |
| PEG 150 (% w/w) | 1 | 1 | 1 | 1 | 1 | 1 |
| Glycerin (ml) | 3 | 3 | 3 | 3 | 3 | 3 |
| Tea tree oil (ml) | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Coconut oil (ml) | 1 | 1 | 1 | 1 | 1 | 1 |
| Aloe vera gel (g) | 8 | 8 | 8 | 8 | 8 | 8 |
| Tween 80 (ml) | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| Propylene glycol (ml) | 5 | 5 | 5 | 5 | 5 | 5 |
| EDTA 0.01, Phenoxyethanol 0.01, Citric acid 0.02 (g) | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 |
| Water (ml) | q. s. | q. s. | q. s. | q. s. | q. s. | q. s. |
In vitro drug release studies
In vitro drug release studies were conducted using Franz diffusion cells with a cellophane membrane. The water-jacketed receptor compartment (22.5 ml capacity) was equipped with a sampling port and thermometer arm. The donor compartment (2 cm internal diameter) was positioned to contact the diffusion medium in the receptor compartment, which contained phosphate buffer (pH 6.8) maintained at 32±1 °C. After membrane equilibration, 1 g of nanoemulgel was applied to the donor compartment. Samples were periodically withdrawn from the receptor compartment with equivalent volume replacement, and analysed using HPLC with a UV detector. Formulation F1 exhibited the fastest drug release profile, attributable to its lower polymer concentration (0.5% HPMC E15).
Kinetic study and mechanism of drug release
The in vitro release study data were subjected to various kinetic models, including zero order, first order, Higuchi, Hixson-Crowell, and Korsmeyer-Peppas equations. This mathematical modelling approach provided insights into the drug release behaviour from the delivery technology.
Globule size analysis for optimized batch F1
The globule size of TANFs was determined using dynamic light scattering (DLS) with a zetasizer. Samples were diluted tenfold with double-distilled water and sonicated to ensure homogenization. The diluted mixtures were analysed in disposable cuvettes at a scattering angle of 90° and temperature of 25 °C. Mean globule size and polydispersity index (PDI) were measured for evaluating formulation stability and performance characteristics.
RESULTS AND DISCUSSION
The evaluation of different formulations (F1–F6) of nanoemulgels for various physicochemical properties, including pH, spreadability, viscosity, colour, texture, phase separation, and homogeneity, provides crucial insights into their quality and usability. The pH of all formulations ranged from 6.2 to 6.3, indicating their compatibility with skin pH, ensuring minimal irritation upon application. The neutral pH (6.2–6.3) and smooth, white appearance of all nanoemulgel formulations indicate they are well-suited for topical application, minimizing potential skin irritation while maintaining aesthetic appeal [35].
F1 exhibited the highest spread ability (4.7±0.05 cm), followed closely by F2 and F4 (4.6±0.3 cm and 4.6±0.05 cm, respectively). In contrast, F6 showed the least spread ability (3.6±0.1 cm), likely due to its higher viscosity. F1, with a viscosity of 1186±0.5 Cp, balanced spread ability and retention properties, making it a promising formulation for further development. Phase separation and homogeneity assessments showed that F1 displayed no phase separation and was homogeneous, indicating a stable formulation. Overall, F1 emerged as the most optimal formulation, balancing desirable pH, spreadability, viscosity, aesthetics and homogeneity (table 2). Regarding spreadability and viscosity, F1 achieved the optimal compromise of sufficient viscosity to ensure good retention alongside the highest spreadability, suggesting it may offer superior ease of application without sacrificing formulation stability [36].
Table 2: pH spread ability, viscosity, colour and texture, phase separation and homogeneity
| Batch code | pH (n=3, mean± SD) | Spread ability (cm) (n=3, mean± SD) |
Viscosity (Cp) (n=3, mean± SD) |
Color and texture | Phase separation and homogeneity |
| F1 | 6.3±0.05 | 4.7±0.05 | 1186±0.5 | White color and smooth texture | No phase separation and homogeneous |
| F2 | 6.3±0.05 | 4.6±0.3 | 1256±0.5 | White Color and Smooth Texture | No Phase separation and Homogeneous |
| F3 | 6.2±0.05 | 4.3±0.3 | 1388±0.5 | White Color and Smooth Texture | No Phase separation and Homogeneous |
| F4 | 6.2±0.05 | 4.6±0.05 | 1230±0.5 | White Color and Smooth Texture | No Phase separation and Homogeneous |
| F5 | 6.2±0.05 | 4.3±0.1 | 1296±1.0 | White Color and Smooth Texture | No Phase separation and Homogeneous |
| F6 | 6.2±0.05 | 3.6±0.1 | 1503±1.5 | White Color and Smooth Texture | No Phase separation and Homogeneous |
*value are expressed as mean±SD (n = 3).
The in vitro cumulative drug release profiles of metronidazole, norfloxacin, and mupirocin were evaluated for six different TANFs (F1–F6) over a 24 h period. The study reveals significant differences in drug release rates across the formulations, highlighting the influence of formulation parameters on drug release behaviour (table 3). The in vitro release profiles for metronidazole, norfloxacin, and mupirocin from F1 demonstrated sustained release over 24 h, signifying that the nanoemulgel matrix effectively supports prolonged drug liberation [37].
Table 3: In vitro cumulative drug release for F1
| Time (hours) | Metronidazole | Norfloxacin | Mupirocin |
| 1 | 3.92 | 3.36 | 4.59 |
| 2 | 9.34 | 8.78 | 10.01 |
| 3 | 16.83 | 16.27 | 17.50 |
| 4 | 24.54 | 23.98 | 25.21 |
| 5 | 32.48 | 31.92 | 33.15 |
| 6 | 42.48 | 41.92 | 43.15 |
| 7 | 52.43 | 51.87 | 53.10 |
| 8 | 63.22 | 62.66 | 63.89 |
| 12 | 74.28 | 73.72 | 74.95 |
| 24 | 90.12 | 89.56 | 90.79 |
*value are expressed as mean (n = 3).
Overall, F1 emerged as the most promising formulation for metronidazole, norfloxacin, and mupirocin delivery, demonstrating consistent release profiles, high cumulative drug release, and stability. These findings underscore the potential of nanoemulgel systems in enhancing the therapeutic efficacy of hydrophobic drugs by providing sustained and targeted drug delivery.
In summary, the release profiles of Metronidazole, Norfloxacin, and Mupirocin showed similar behaviour, with all formulations following the Higuchi diffusion model and first-order kinetics. These results confirm that the drug release is primarily diffusion-controlled, with the rate governed by the concentration of the drug in the system. This controlled and sustained release behaviour highlights the potential therapeutic advantages of these formulations in providing prolonged drug action, improving patient compliance, and enhancing therapeutic efficacy (table 4). Kinetic modeling showed that drug release followed diffusion-controlled mechanisms, as indicated by high correlation values in the Higuchi and related models, pointing to release governed predominantly by diffusion through the gel matrix [38].
Table 4: Kinetic study and mechanism of drug release of metronidazole (M), norfloxacin (N), and mupirocin (Mu)
| Time (h) | Log t | Log% % cumulative drug release (M) | Cube root % % Qt (M) | Log% % cumulative drug release (N) | Cube root% Qt (N) |
| 1.0 | 0.0 | 1.6 | 3.42 | 1.54 | 3.27 |
| 2.0 | 0.3 | 1.78 | 3.91 | 1.75 | 3.83 |
| 3.0 | 0.48 | 1.86 | 4.16 | 1.83 | 4.08 |
| 4.0 | 0.6 | 1.9 | 4.31 | 1.88 | 4.24 |
| 5.0 | 0.7 | 1.95 | 4.48 | 1.94 | 4.43 |
| 6.0 | 0.78 | 1.96 | 4.51 | 1.95 | 4.48 |
| 7.0 | 0.85 | 1.97 | 4.55 | 1.96 | 4.51 |
| 8.0 | 0.9 | 1.98 | 4.58 | 1.97 | 4.55 |
| 12.0 | 1.08 | 1.99 | 4.61 | 1.99 | 4.59 |
| 24.0 | 1.38 | 2.0 | 4.64 | 2.0 | 4.63 |
*value are expressed as mean (n = 3).
Table 5: Diffusion kinetics of metronidazole, norfloxacin, and mupirocin
| Diffusion Kinetics | R2 | ||
| Metronidazole | Norfloxacin | Muprocin | |
| Zero-order plot | 0.843 | 0.843 | 0.833 |
| First Order Plot | 0.673 | 0.693 | 0.629 |
| Korsemeyer-Peppas plot | 0.943 | 0.947 | 0.941 |
| Hixson-crowell plot | 0.962 | 0.983 | 0.961 |
| Higuchi plot | 0.983 | 0.987 | 0.986 |
The correlation coefficient (R2) of the Higuchi model was found to be 0.983, 0.987, and 0.986, respectively, for meteronidazole, Norfloxacin, and muprocinis, slightly higher when compared to the other plots. Hence, this indicates that the release data of selected batch F1 follows the Higuchi model because the value of R2 is greater in this model for drug diffusion (table 5).
Table 6: Drug content (%) of metronidazole (M), norfloxacin (N), and mupirocin (Mu) in different formulations
| Formulation | Metronidazole (%) | Norfloxacin (%) | Mupirocin (%) |
| F1 | 96.45±0.28 | 95.89±0.34 | 96.12±0.26 |
| F2 | 96.73±0.31 | 96.21±0.29 | 96.45±0.33 |
| F3 | 97.01±0.25 | 96.54±0.27 | 96.88±0.30 |
| F4 | 95.87±0.22 | 95.60±0.24 | 95.92±0.28 |
| F5 | 96.12±0.30 | 95.98±0.33 | 96.20±0.25 |
| F6 | 96.38±0.27 | 96.10±0.31 | 96.50±0.29 |
*value are expressed as mean±SD (n = 3).
The drug content analysis of the six nanoemulgel formulations (F1–F6) revealed consistent and uniform drug distribution, with all formulations exhibiting drug content above 95% for metronidazole, norfloxacin, and mupirocin. This high drug retention indicates effective incorporation of the antibiotics into the nanoemulgel matrix during formulation. Among all, formulation F3 showed the highest drug content values for all three drugs, likely due to optimal polymer concentration and homogenous mixing. The low standard deviation (SD) values further confirm the reproducibility and reliability of the method used. These findings support the stability of the nanoemulgel system and validate its suitability for topical application in treating chronic wounds with polymicrobial infections (table 6). High and consistent drug content (>95 %) across formulations reflects efficient drug incorporation and reproducibility of the formulation process, with F3 showing slightly higher loading that may reflect subtle compositional differences [39].
Table 7: Particle size analysis of the prepared nanogel formulations
| Batch code | Particle size (nm)* |
| F1 | 223.1±3.1 |
| F2 | 256.34±2.61 |
| F3 | 285.58±1.29 |
| F4 | 266.81±1.32 |
| F5 | 293.72±3.64 |
| F6 | 326.62± 2.36 |
*value are expressed as mean±standard deviation (n=3).
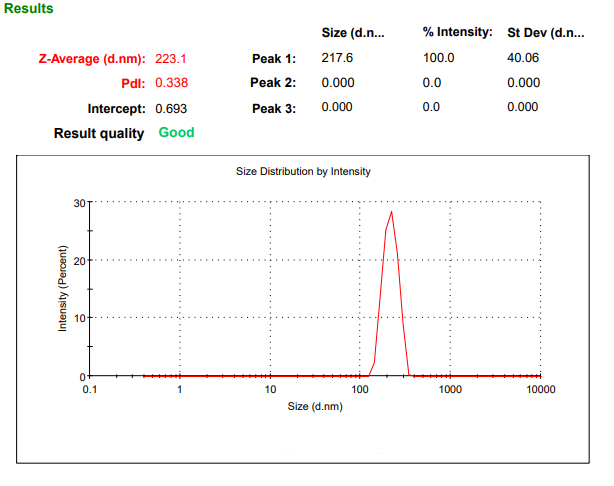
Fig. 3: Particle size analysis of batch F1
The particle size analysis of the prepared TANFs revealed a range of sizes across the different batches, highlighting variations in the formulation parameters. Batch F1 exhibited the smallest particle size of 223.1±3.1 nm, indicating its potential for enhanced stability and efficient drug delivery (table 7, fig. 3). Smaller particle sizes support better penetration and sustained drug release, underscoring the significance of precise control in nanoemulgel preparation. Particle-size analysis revealed that F1 had the smallest nanoemulsion droplets (\~223 nm), which is advantageous for dermal delivery efficiency and formulation stability [40]. Together, these characteristics underscore that F1 is the most promising among the evaluated nanoemulgels. To support its development further, subsequent studies should include ex vivo skin permeation, antimicrobial efficacy testing against polymicrobial wound pathogens, and comprehensive stability profiling under accelerated conditions [41].
Future perspectives
While the proposed work presents a drug device combination with a nanoemulgel matrix to deliver three antibiotics for the treatment of chronic wounds, like as diabetic and burn patients, where there is a presence of complex microbial manifestation. Future research will focus on conducting cytotoxicity studies to ensure the formulation's safety for dermal application. Additionally, irritation and sensitization tests will be performed to assess its compatibility with the skin and to identify any potential adverse reactions. These studies are essential for establishing the formulation's clinical relevance and safety profile. Moreover, advanced investigations such as in vivo efficacy studies, long-term stability assessments under varying environmental conditions, and large-scale production feasibility tests will be undertaken to enhance the formulation's translational potential. It is important to note that the current laboratory data serves as an ideation-stage exploration, providing a foundation for subsequent rigorous preclinical and clinical validations. This progressive approach will ensure the formulation meets regulatory standards and effectively addresses the unmet needs in the treatment of chronic diabetic wounds.
CONCLUSION
In conclusion, this research presents a novel nanoemulgel formulation incorporating Metronidazole, Norfloxacin, and Mupirocin, designed to address the challenges associated with the treatment of chronic diabetic wounds. The formulation demonstrated remarkable pharmaceutical properties, including high drug release efficiency, stability, and excellent spreadability, making it a promising candidate for topical antibiotic therapy. The use of HPMC E15 as a gelling agent and the incorporation of strategic permeation enhancers like tea tree oil and coconut oil contributed to enhanced skin permeability and a dual-controlled drug release mechanism. The proposed formulation effectively combines the benefits of nanoemulsion and gels, achieving improved solubility, stability, and bioavailability of the encapsulated antibiotics. Moreover, the adherence to Higuchi release kinetics and the demonstrated antihistaminic effects underline the formulation's potential for dermatological applications. By addressing the limitations of conventional topical delivery systems, this study paves the way for the development of innovative and patient-friendly therapeutic solutions for chronic wound management. Future investigations into the clinical efficacy, safety, and large-scale production of this nanoemulgel will further validate its potential as a ground-breaking approach in topical drug delivery.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
Ms. Nipa Thacker is the research scholar who was responsible for the research work carried out, drafting the manuscript, and compiling the experimental data. Dr. Sunny Rathee contributed by editing the manuscript and providing critical revisions for intellectual content. Prof. Richa Dayaramani offered continuous guidance throughout the study and critically reviewed the final version of the manuscript. All authors have read and approved the final manuscript.
CONFLICT OF INTERESTS
Declared none
REFERENCES
Anand K, Ray S, Rahman M, Shaharyar A, Bhowmik R, Bera R. Nano-emulgel: emerging as a smarter topical lipidic emulsion-based nanocarrier for skin healthcare applications. Recent Pat Anti-Infect Drug Discov. 2019;14(1):16-35. doi: 10.2174/1574891X14666190717111531, PMID 31333141.
Donthi MR, Munnangi SR, Krishna KV, Saha RN, Singhvi G, Dubey SK. Nanoemulgel: a novel nano carrier as a tool for topical drug delivery. Pharmaceutics. 2023 Jan 3;15(1):164. doi: 10.3390/pharmaceutics15010164, PMID 36678794, PMCID PMC9863395.
Yu M, Ma H, Lei M, Li N, Tan F. In vitro/in vivo characterization of nanoemulsion formulation of metronidazole with improved skin targeting and anti-rosacea properties. Eur J Pharm Biopharm. 2014;88(1):92-103. doi: 10.1016/j.ejpb.2014.03.019, PMID 24704200.
Najm MB, Rawas Qalaji M, Assar NH, Yahia R, El Hosary RE, Ahmed IS. Optimization characterization and in vivo evaluation of mupirocin nanocrystals for topical administration. Eur J Pharm Sci. 2022;176:106251. doi: 10.1016/j.ejps.2022.106251, PMID 35788029.
Dua K, Malipeddi VR, Madan J, Gupta G, Chakravarthi S, Awasthi R. Norfloxacin and metronidazole topical formulations for effective treatment of bacterial infections and burn wounds. Interv Med Appl Sci. 2016 Jun 30;8(2):68-76. doi: 10.1556/1646.8.2016.2.4, PMID 28386462.
Dave V, Yadav RB, Kushwaha K, Yadav S, Sharma S, Agrawal U. Lipid polymer hybrid nanoparticles: development and statistical optimization of norfloxacin for topical drug delivery system. Bioact Mater. 2017 Dec 1;2(4):269-80. doi: 10.1016/j.bioactmat.2017.07.002, PMID 29744436.
Choudhury H, Gorain B, Pandey M, Chatterjee LA, Sengupta P, Das A. Recent update on nanoemulgel as topical drug delivery system. J Pharm Sci. 2017 Jul;106(7):1736-51. doi: 10.1016/j.xphs.2017.03.042, PMID 28412398.
Aithal GC, Narayan R, Nayak UY. Nanoemulgel: a promising phase in drug delivery. Curr Pharm Des. 2020;26(2):279-91. doi: 10.2174/1381612826666191226100241, PMID 31878849.
Ullah N, Amin A, Farid A, Selim S, Rashid SA, Aziz MI. Development and evaluation of essential oil-based nanoemulgel formulation for the treatment of oral bacterial infections. Gels. 2023 Mar 21;9(3):252. doi: 10.3390/gels9030252, PMID 36975701, PMCID PMC10048686.
Singh I, Kaur P, Kaushal U, Kaur V, Shekhar N. Essential oils in treatment and management of dental diseases. Biointerf Res Appl Chem. 2022;12(6):7267-86. doi: 10.33263/briac126.72677286.
Ma Q, Zhang J, Lu B, Lin H, Sarkar R, Wu T. Nanoemulgel for improved topical delivery of desonide: formulation design and characterization. AAPS Pharm Sci Tech. 2021;22(5):163. doi: 10.1208/s12249-021-02035-5, PMID 34031790.
Azhar M, Amul Mishra. Review of nanoemulgel for treatment of fungal infections. Int J Pharm Pharm Sci. 2024 May 21;16(9):8-17. doi: 10.22159/ijpps.2024v16i9.51528.
Hmingthansanga V, Singh N, Banerjee S, Manickam S, Velayutham R, Natesan S. Improved topical drug delivery: role of perme
ation enhancers and advanced approaches. Pharmaceutics. 2022 Dec 15;14(12):2818. doi: 10.3390/pharmaceutics14122818, PMID 36559311.
Lane ME. Skin penetration enhancers. Int J Pharm. 2013 Apr 15;447(1-2):12-21. doi: 10.1016/j.ijpharm.2013.02.040, PMID 23462366.
Chellapa P, Eid A, Elmarzugi N. Preparation and characterization of virgin coconut oil nanoemulgel. 2015;7(9):787-93.
Bujubarah MM, Elsewedy HS, Shehata TM, Soliman WE. Formulation by design of an innovative tea tree oil nanoemulgel incorporating mupirocin for enhanced wound healing activity. Appl Sci. 2023;13(24):13244. doi: 10.3390/app132413244.
Silva RC, Trevisan MG, Garcia JS. Characterization and drug excipient compatibility study of bromopride by DSC, FTIR and HPLC. J Therm Anal Calorim. 2024;149(17):9333-42. doi: 10.1007/s10973-024-13392-1.
Bruni G, Amici L, Berbenni V, Marini A, Orlandi A. Drug excipient compatibility studies search of interaction indicators. J Therm Anal Calorim. 2002 May 1;68(2):561-73. doi: 10.1023/A:1016052121973.
Ahmad J, Gautam A, Komath S, Bano M, Garg A, Jain K. Topical nano-emulgel for skin disorders: formulation approach and characterization. Recent Pat Antiinfect Drug Discov. 2019 May 1;14(1):36-48. doi: 10.2174/1574891X14666181129115213, PMID 30488798.
Gadkari PN, Patil PB, Saudagar RB. Formulation development and evaluation of topical nanoemulgel of tolnaftate. J Drug Deliv Ther. 2019 Mar 2;9(2):208-13. doi: 10.22270/jddt.v9i2-s.2495.
Algahtani MS, Ahmad MZ, Shaikh IA, Abdel Wahab BA, Nourein IH, Ahmad J. Thymoquinone loaded topical nanoemulgel for wound healing: formulation design and in vivo evaluation. Molecules. 2021 Jun 24;26(13):3863. doi: 10.3390/molecules26133863, PMID 34202733.
Bashir M, Ahmad J, Asif M, Khan SU, Irfan M, Y Ibrahim A. Nanoemulgel an innovative carrier for diflunisal topical delivery with profound anti-inflammatory effect: in vitro and in vivo evaluation. Int J Nanomedicine. 2021;16:1457-72. doi: 10.2147/IJN.S294653, PMID 33654396.
Priyadarshini P, Karwa P, Syed A, Asha AN. Formulation and evaluation of nanoemulgels for the topical drug delivery of posaconazole. J Drug Deliv Ther. 2023;13(1):33-43. doi: 10.22270/jddt.v13i1.5896.
Tashtoush BM, Jacobson EL, Jacobson MK. Validation of a simple and rapid HPLC method for determination of metronidazole in dermatological formulations. Drug Dev Ind Pharm. 2008 Jan 1;34(8):840-4. doi: 10.1080/03639040801928598, PMID 18618307.
Echevarria L, Blanco Prieto MJ, Campanero MA, Santoyo S, Ygartua P. Development and validation of a liquid chromatographic method for in vitro mupirocin quantification in both skin layers and percutaneous penetration studies. J Chromatogr B Analyt Technol Biomed Life Sci. 2003 Nov 5;796(2):233-41. doi: 10.1016/j.jchromb.2003.07.011, PMID 14581064.
Chierentin L, Salgado HR. Review of properties and analytical methods for the determination of norfloxacin. Crit Rev Anal Chem. 2016 Jan 2;46(1):22-39. doi: 10.1080/10408347.2014.941456, PMID 26398574.
Donthi MR, Saha RN, Singhvi G, Dubey SK. Dasatinib-loaded topical nano-emulgel for rheumatoid arthritis: formulation design and optimization by QbD, in vitro, ex vivo, and in vivo evaluation. Pharmaceutics. 2023;15(3):736. doi: 10.3390/pharmaceutics15030736, PMID 36986597.
Barkat MA, Hasan N, Hassan MohdZ, Asiri YI, Nadaf A, Ahmad FJ. Formulation development of methotrexate lipid-based nanogel for treatment of skin cancer. Colloids Surf A Physicochem Eng Aspects. 2024 May 5;688:133571, doi: 10.1016/j.colsurfa.2024.133571.
Gaber DA, Alsubaiyel AM, Alabdulrahim AK, Alharbi HZ, Aldubaikhy RM, Alharbi RS. Nano-emulsion-based gel for topical delivery of an anti-inflammatory drug: in vitro and in vivo evaluation. Drug Des Dev Ther. 2023;17:1435-51. doi: 10.2147/DDDT.S407475, PMID 37216175.
Sghier K, Mur M, Veiga F, Paiva Santos AC, Pires PC. Novel therapeutic hybrid systems using hydrogels and nanotechnology: a focus on nanoemulgels for the treatment of skin diseases. Gels. 2024 Jan 6;10(1):45. doi: 10.3390/gels10010045, PMID 38247768, PMCID PMC10815052.
Manure Hirakant S, Nagoba Shivappa N. Design development and evaluation of novel nanoemulgel for topical drug delivery system. J Biomech Sci Eng. 2023 Jul:84-99.
Pereira GF, Balmith M, Nell M. The efficacy of honey as an alternative to standard antiseptic care in the treatment of chronic pressure ulcers and diabetic foot ulcers in adults. Asian J Pharm Clin Res. 2021;5(6):37-47. doi: 10.22159/ajpcr.2021.v14i11.42560.
Thakre S Chanchlesh Dehariya, Shivam Upadhyay, Shailendra Singh Nargesh, Manisha Singh. A prospective study of epidemiological factors and outcome in patients of diabetic foot wound attending J. A Group of Hospitals: A Tertiary Care Centre. 2023 Jun;16(6):149-52. doi: 10.22159/ajpcr.2023.v16i6.48316.
Manasa MT, Ramanamurthy KV, Bhupathi PA. Electrospun nanofibrous wound dressings: a review on chitosan composite nanofibers as potential wound dressings. Int J App Pharm. 2023 Jul;15(4):1. doi: 10.22159/ijap.2023v15i4.47912.
Alhasso B, Ghori MU, Conway BR. Development of nanoemulsions for topical application of mupirocin. Pharmaceutics. 2023;15(2):378. doi: 10.3390/pharmaceutics15020378, PMID 36839700.
Ullah N, Amin A, Farid A, Selim S, Rashid SA, Aziz MI. Development and evaluation of essential oil based nanoemulgel formulation for the treatment of oral bacterial infections. Gels. 2023 Mar 21;9(3):252. doi: 10.3390/gels9030252, PMID 36975701.
Donthi MR, Munnangi SR, Krishna KV, Saha RN, Singhvi G, Dubey SK. Nanoemulgel: a novel nano carrier as a tool for topical drug delivery. Pharmaceutics. 2023 Jan 3;15(1):164. doi: 10.3390/pharmaceutics15010164, PMID 36678794.
Yetukuri K, Umashankar MS. Development and optimization of Kunzea ericoides nanoemulgel using a quality by design approach for transdermal anti-inflammatory therapy. Gels. 2025 May 27;11(6):400. doi: 10.3390/gels11060400, PMID 40558699.
Manure Hirakant S, Nagoba Shivappa N. Design development and evaluation of novel nanoemulgel for topical drug delivery system. J Biomech Sci Eng. 2023:84-99.
Gaddala P, Choudhary S, Sethi S, Sainaga Jyothi VG, Katta C, Bahuguna D. Etodolac utility in osteoarthritis: drug delivery challenges topical nanotherapeutic strategies and potential synergies. Ther Deliv. 2024 Dec 1;15(12):977-95. doi: 10.1080/20415990.2024.2405456, PMID 39345034.
Rehman SU, Khan NR, Ullah M, Shah SU, Rehman AU, Jamal Q. Nanoemulgel mediated enhanced skin curcumin penetration/retention for local treatment of cutaneous leishmaniasis: in vitro and in vivo assessment. Drug Dev Ind Pharm. 2025 Apr 3;51(4):354-64. doi: 10.1080/03639045.2025.2473495, PMID 40022627.