
Int J Pharm Pharm Sci, Vol 17, Issue 11, 25-31Original Article
DEVELOPMENT OF BUCCAL MUCOADHESIVE FILM FOR RAPID DELIVERY OF NARATRIPTAN
MANTHASA1*, FARHEEN FAZEELAT2, ALVIA ANJUM3
1,2*Department of Pharmaceutics, Moonray Institute of Pharmaceutical Sciences, Raikal, RR-509216, India. ³Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Moonray Institute of Pharmaceutical Sciences, Raikal, RR-509216, India
*Corresponding author: Manthasa; *Email: manthashaik@gmail.com
Received: 14 Jul 2025, Revised and Accepted: 02 Oct 2025
ABSTRACT
Objective: To prepare matrix-type buccal therapeutic systems of naratriptan using various polymers as matrix formers. Formulated buccal films were characterized for physicochemical properties.
Methods: Naratriptan mucoadhesive buccal films were developed by using solvent casting method (evaluation parameters are film uniformity and thickness, weight variation, moisture content, folding endurance) and by direct milling method (drug content uniformity, swelling behavior, in vitro release studies, stability studies).
Results: Results revealed that prepared patches showed good physical characteristics, no drug-polymer interaction was observed. The in vitro release study revealed that F1 formulation showed maximum release of 94.62% in 8 h. The absorption maxima of naratriptan is at 275 nm. The predominant release mechanism of drug through the fabricated matrices was believed to be by diffusion mechanism and the percentage drug release was found to be decreasing from 94.62% to 91.20%.
Conclusion: Based upon the in vitro dissolution data the F1 formulation was concluded as optimized formulation.
Keywords: Buccal patch, Buccal delivery system, Naratriptan, Natural and synthetic polymers, Solvent casting technique, Diffusion mechanism
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijpps.2025v17i11.56075 Journal homepage: https://innovareacademics.in/journals/index.php/ijpps
INTRODUCTION
The oral route has been seen as a popular and an attractive route for the drug administration by many researchers. Reasons such as ease of administration, better patient acceptance and compliance, easy preparation of dosage forms and the versatility to administer different type and quantities of drugs [1]. However, there are number of disadvantages associated with the oral route of drug administration, like high first-pass metabolism, ability to cause gastric irritation by drugs, possibilities of degradation of drugs in gastric fluid and delayed onset of action in some cases makes oral mucosal route an important alternative for drug administration. The main feature of the oral mucosal route is to administer the drug into the systemic circulation by diffusing drug through the oral mucosa. The sublingual and buccal mucosa are two routes with which the drug can be administered into systemic circulation [2]. The oral mucosal route is very attractive approach for the systemic administration of macromolecules like proteins and peptides. These macromolecules exhibit superior avoidance of first-pass hepatic metabolism of drugs when administered via buccal mucosal route and can help in improving bioavailability of drugs. Drugs administered via buccal mucosal route enter into jugular vein followed by the entry into systemic circulation. This means the buccal mucosal route offers a distinctive advantage for the absorption of drugs which are cleared largely by the liver [3].
The drug used in this work is naratriptan, which is a selective serotonin receptor agonist. It is available in the form of white crystalline powder and has a characteristic odor. naratriptan is used to treat acute migraine headaches in adults. It belongs to the group of medicines called triptans. The aim of this study was to formulate and evaluate the mucoadhesive film of naratriptan.
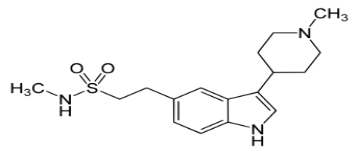
IUPAC Name: N-methyl-2-[3-(1-methylpiperidin-4-yl)-1H-indol-5yl] ethanesulfonamide
MATERIALS AND METHODS
Chemicals and reagents used: naratriptan (heterolabs, hyd), sodiumcarboxy methylcellulose (synpharma research labs, hyd), methanol (Synpharma Research Labs, hyd), polyethylene glycol (Synpharma Research Labs, hyd), dimethylsulphoxide (synpharma research labs, hyd), aspartame (synpharma research labs, hyd).
Equipment used: Digital weighing machine (Shimadzu aty 244), UV-Visible double beam spectrophotometer (lab India UV visible double beam spectrophotometer), franz diffusion cell (AR chemicals, hyd), magnetic stirrer (Erweka), bath sonicator (Wensar) [4].
Preformulation studies
Preformulation involves the application of biopharmaceutical principles and the physicochemical parameters of drug substance were characterized with the goal of designing optimum drug delivery system. Characterization of drug is very important step in the preformulating phase of product development followed by studying the properties of excipients and their compatibility [5].
Preparation of phosphate buffer pH 6.8
28.85 g of di-sodium hydrogen orthophosphate and 11.45 g of potassium dihydrogen phosphate was weighed to it sufficient water was added to get 1000 ml and the pH was altered to 6.8 with phosphoric acid [6].
Standard graph of naratriptan in phosphate buffer pH6.8
Standard stock solution of naratriptan (1 mg/ml) was prepared by dissolving 100 mg of naratriptan in 100 ml of methanol. Diluting the standard stock solution with phosphate buffer 6.8, solution of 100 µg/ml concentration was prepared. From this solution serial dilutions were made with phosphate buffer 6.8 to get 10, 20, 30, 40, 50 µg/ml concentrations. These solutions were checked for the absorbance using UV-Visible spectrophotometer at λ max against phosphate buffer 6.8 as blank and standard graph was plotted by taking concentration on X-axis and absorbance on Y-axis [7].
Drug-excipient compatibility study
FTIR Spectral Studies FTIR spectra of pure drug, polymers and their physical mixtures (stored at 40±2oC/75%±5% RH for 2 mo) were recorded. The samples were prepared by potassium bromide disc method and scanned for absorbance [8].
Formulation design of naratriptan buccal films
Method of preparation
Solvent casting: All the patch components, including the drug, are mixed in an organic solvent and coated onto a release liner. Once the solvent evaporates, a thin layer of protective backing material is applied to the coated release liner, creating a laminate. This laminate is then cut into films of the desired size and shape [9].
Direct milling: This method involves the production of films without using solvents, making it a solvent-free approach. The drug and excipients are mixed mechanically through techniques like direct milling or kneading, typically without any liquids. The blended material is then rolled onto a release liner to achieve the required thickness [10]. An impermeable backing membrane can be included to guide drug release, preventing drug loss, and maintain the device’s stability during use. While the performance of films made with solvent-based and solvent-free methods is largely comparable, the solvent-free technique is favoured due to its elimination of residual solvents and associated health risks [11].
Table 1: Formulation design of naratriptan
| F. No. | Drug (mg) | Na CMC (mg) | Aspartame (mg) | PEG (ml) | DMSO (ml) |
| F1 | 2 | 25 | 2 | 1 | 0.1 |
| F2 | 2 | 50 | 2 | 1 | 0.1 |
| F3 | 2 | 75 | 2 | 1 | 0.1 |
| F4 | 2 | 100 | 2 | 1 | 0.1 |

Fig. 1: Solvent casting method
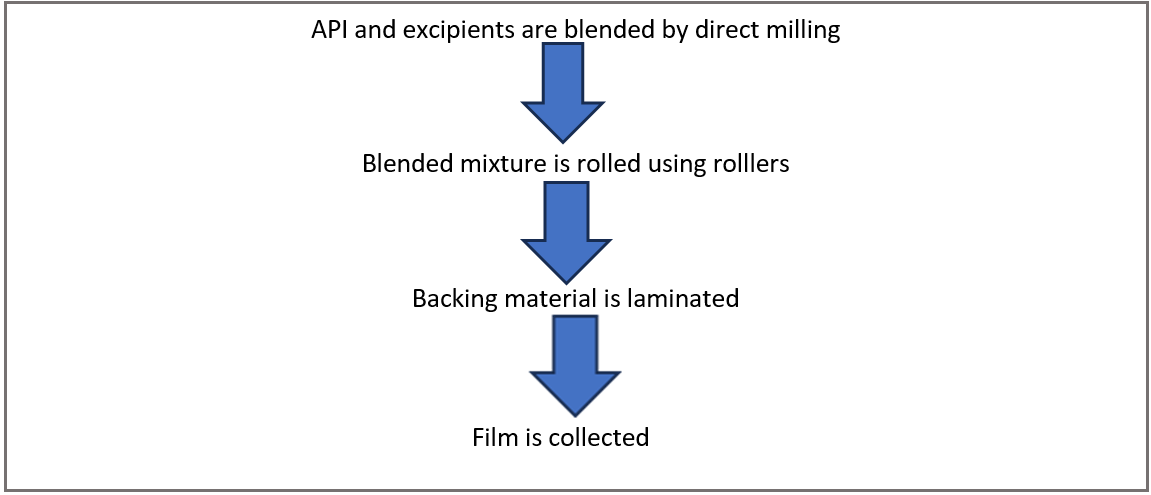
Fig. 2: Direct milling method
Characterization of buccal formulation
Physico-chemical evaluation
Physical appearance
All the formulated naratriptan films were observed for colour, clarity, flexibility, and smoothness [12].
Folding endurance
Buccal films folding endurance was estimated by frequently double over at the same place till it broke. The number of times the films could be folded at the same place without breaking is the folding endurance [13].
Thickness of the films
The thickness of each film was measured by using screw gauze. Buccal films thickness was estimated at various sites on each film and the average thickness of the buccal film was capture as the thickness of the film [14].
Weight uniformity
The formulated buccal films are to be dried at 60 °C for 6 h before trial. A identify the area of 4.52 cm2 of films is to be cut in different parts of the film and weigh in digital balance [15].
Drug content
The medicated film (2 cm diameter), without backing membrane was allowed to dissolve in 10 ml of simulated saliva solution (pH 6.8) for 2-3 h under occasional shaking. The resultant solution was filtered through 0.45 µm filter paper and after suitable dilution, the amount of drug present in the film was determined spectrophotometrically at 275 nm (Shimadzu 1800, Japan) [16].
Swelling behaviour
The initial diameter of the original film (2 cm diameter), without backing membrane was determined. Then the sample was allowed to swell on the surface of an agar plate (prepared as described under measurement of surface pH section) kept in an incubator maintained at 37±1 °C. Measurement of the diameter of the swollen film was carried out at predetermined time intervals for 90 min [17].
Moisture absorption studies
The buccal films were weighed exactly and placed in a desiccator containing aluminium chloride to maintain 79.50% RH. After 3 days, the films were taken out and weighed [18].
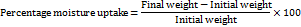
Moisture loss studies
Three films were weighed separately and kept in a desiccator contains calcium chloride at 37 C for 24 h. Then the last weight was noted when there was no further change in the weight of the film. The percentage of moisture loss was calculated using the following formula [19].

In vitro release study
The release rate of the drug was determined by using franz diffusion cell apparatus temperature maintained at 37±0.5 C and stirred at a rate of 200 rpm. The vessel containing 10 ml of phosphate buffer pH 6.8 phosphate buffer solution. Aliquots of 1 ml of samples were withdrawn at various time meanwhile and then analysed using a UV Spectrophotometer [20].
% release rate of drug was determined using the following formula.
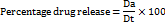
Where, Dt = Total amount of the drug in the films
Da = The amount of drug released
The study was carried out using phosphate buffer (pH 7.4) as the dissolution medium, maintained at a temperature of 37±0.5 °C, with the apparatus operated at 200 rpm. Samples were withdrawn at predetermined time intervals of 1, 2, 3, 4, 5, 6, 7, and 8 h.

Fig. 3: The study was carried out using phosphate buffer
Stability studies
Selected films were subjected to accelerated stability testing by wrapping them in aluminium foil and packing them in glass vials. These films were kept in an incubator maintained at 37±0.5 °C and 75±5% RH for 6 mo. The film was stable only up to 37 °C while conducting the stability studies [21]. When the films were kept at 40 °C, the films become pliable and showed instability. Changes in the appearance, residence time, in vitro drug release and drug content of the stored films were investigated after 3 mo [22].
RESULTS AND DISCUSSION
Active pharmaceutical ingredient characterization
Physical properties
The colour, odour, taste of the drug was recorded using descriptive terminology.
Solubility studies
Slightly Soluble in methanol and dimethylsulphoxide.
Melting point determination
The melting point of the drug is determined as 238 °C.
Determination of λ max using UV-Visible spectrophotometer
Naratriptan exhibits absorption maxima at 275 nm in phosphate buffer 6.8.
Determination of absorption maxima (λmax) for naratriptan
A 10mcg/ml standard solution of naratriptan was scanned on a double-beam spectrophotometer against respective media blanks. An absorption maximum (λmax) of 275 nm was obtained for all solutions and was selected to prepare standard curve.
These characteristics shows that good polymer selection will be essential to improve naratriptan buccal delivery [23].
Compatibility studies of drug and polymers
All these peaks have appeared in formulation and physical mixture, indicating no chemical interaction between naratriptan and polymer. It also confirmed that the stability of drug during microencapsulation process suggesting that formulations ensure drug stability within mucoadhesive films [24].
Characterization of buccal films
Physical appearance and surface texture of buccal films: These parameters were checked simply with visual inspection of films and by feel or touch. The observation reveals that the films are having smooth surface and they are elegant in appearance.
Weight uniformity of buccal films: The weight of the films was determined using digital balance and the average weight of all films.
Thickness of buccal films: The thickness of the films was measured using screw gauge and the average thickness of all films.
Table 2: Calibration curve of naratriptan
| S. No. | Concentration (mcg/ml) | Absorbance |
| 1 | 0 | 0 |
| 2 | 10 | 0.132 |
| 3 | 20 | 0.234 |
| 4 | 30 | 0.327 |
| 5 | 40 | 0.435 |
| 6 | 50 | 0.543 |

Fig. 4: Calibration curve of naratriptan
Table 3: Characteristic peaks for naratriptan
| S. No. | Characteristic peaks | Frequency range (cm-1) | Frequency (cm-1) |
| 1 | OH stretching | 3500-3000 | 3452.70 |
| 2 | OH Bending | 3000-2750 | 2870.17 |
| 3 | C-H stretching | 2000-1500 | 1737.92 |
| 4 | C=O stretching | 1250-1000 | 1105.25 |
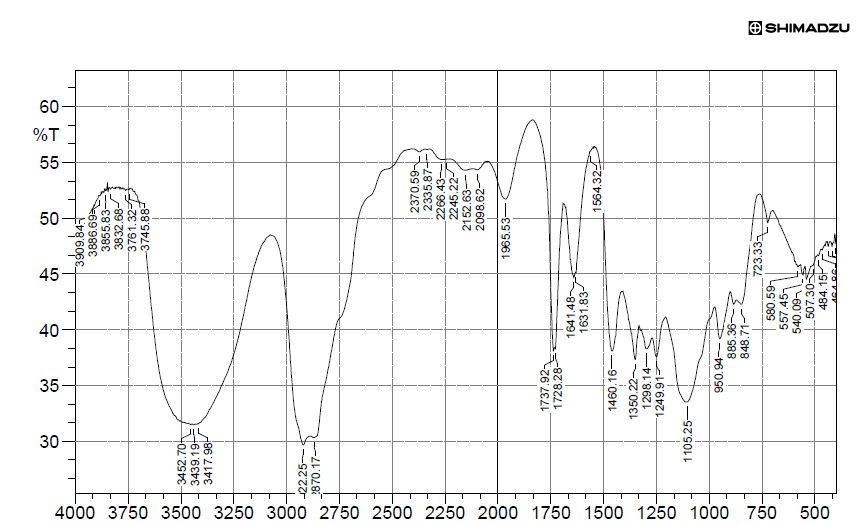
Fig. 5: FTIR studies of naratriptan

Fig. 6: FTIR Studies of physical mixture of excipients
Table 4: Characteristic peaks for physical mixture of excipient
| S. No. | Characteristics peak | Frequency range (cm-1) | Frequency (cm-1) |
| 1 | OH stretching | 4000-3500 | 3832.68 |
| 2 | OH bending | 3500-3000 | 3421.83 |
| 3 | C=O stretching | 2500-2000 | 2359.02 |
Folding endurance of buccal films
The folding endurance gives the idea of flexible nature of films. The folding endurance was measured manually, films were folded repeatedly till it broke, and it was considered as the end point. The folding endurance was found optimum and the films exhibited good physical and mechanical properties and the average folding endurance of all films.
Drug content uniformity of buccal films
Naratriptan buccal films prepared with various polymers were subjected to the valuation for uniform dispersion of drug throughout the patch. In each case three films were used and the average drug content was calculated.
Percentage moisture loss
The moisture content in the buccal films ranged from 8.12 to 8.23 %. The moisture content in the formulations was found to be increased by increase in the concentration of polymers.
Percentage moisture absorption
The moisture absorption in the buccal films ranged from 8.82 to 9.98 %.
Swelling index
The swelling index in the buccal films ranged from 14.82to 15.70 %.
These characteristics highlight the design focus of our formulation and studies confirmed smooth surface, adequate flexibility, and suitable swelling index, indicating that the films possess desirable mechanical strength and mucoadhesive properties for rapid and effective buccal delivery of triptans [25].
Stability studies
Optimized formulation F1 was selected for accelerated stability studies as per ICH guidelines. The films were observed for colour, appearance and flexibility for a period of three months. Percentage cumulative drug release of the formulation was found to be decreasing. This decrease may be attributed to the harsh environment (40 °C) maintained during the studies. Our formulation exhibited better stability under ICH conditions demonstrating improved storage stability, supporting its potential as a more reliable system for rapid delivery of naratriptan in migraine therapy when compared to conventional oral drugs [26].
Table 5: Physiochemical evaluation data of naratriptan buccal films
| F. code | F1 | F2 | F3 | F4 |
| Thickness(mm) | 0.31±0.01 | 0.32±0.01 | 0.37±0.01 | 0.29±0.01 |
| Weight variation (mg) | 40.25±0.15 | 38.1±0.1 | 35.18±0.23 | 30.25±0.25 |
| Drug content uniformity | 95.25±0.28 | 94.88±0.17 | 92.72±0.23 | 93.21±0.22 |
| Folding endurance | 58±0.5 | 59±0.5 | 67±0.5 | 64±0.5 |
| %moisture loss | 8.14±0.16 | 8.12±0.17 | 8.23±0.15 | 8.20±0.23 |
| %moisture absorption | 9.98±0.10 | 8.82±0.21 | 9.75±0.23 | 9.15±0.18 |
| Swelling index% | 14.88±0.27 | 14.82±0.21 | 15.17±0.31 | 15.70±0.36 |
Data is expressed as mean± SD; n=3. (The table here discusses about the drug naratriptan and therefore underweight variation, as the polymer content increases the drug content keeps on decreasing when we weigh 4.52 cm2 of film.), (Swelling index: There was an transcription error in entering the values)
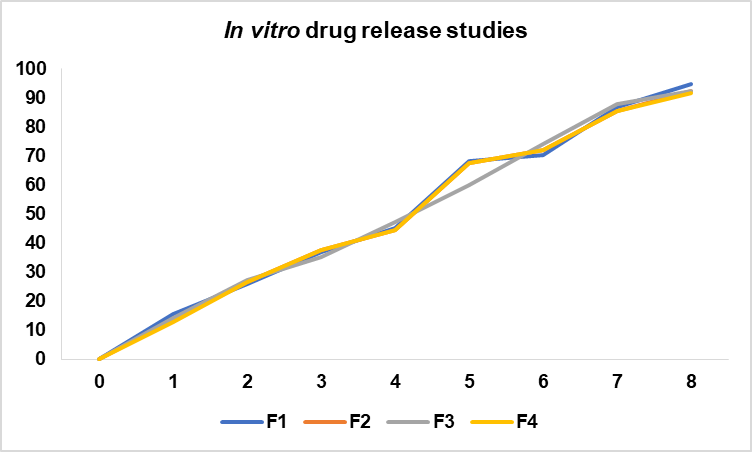
Fig. 7: In vitro drug release
Table 6: In vitro release data of film F1 to F4 (Data table for drug release curves)
| Time (h) | F1 | F2 | F3 | F4 |
| 0 | 0 | 0 | 0 | 0 |
| 1 | 15.63±0.15 | 13.58±0.17 | 14.25±0.15 | 12.72±0.12 |
| 2 | 25.89±0.19 | 26.50±0.10 | 27.45±0.15 | 26.50±0.10 |
| 3 | 36.87±0.16 | 37.70±0.15 | 35.12±0.17 | 37.70±0.15 |
| 4 | 45.23±0.13 | 44.50±0.15 | 47.16±0.16 | 44.50±0.15 |
| 5 | 68.35±0.15 | 67.65±0.25 | 59.82±0.12 | 67.65±0.15 |
| 6 | 70.34±0.14 | 71.98±0.18 | 74.23±0.18 | 71.98±0.18 |
| 7 | 86.77±0.17 | 85.32±0.22 | 87.90±0.10 | 85.32±0.22 |
| 8 | 94.62±0.17 | 92.12±0.17 | 92.40±0.15 | 91.53±0.15 |
Data is expressed as mean±SD; n = 3.
Table 7: Stability studies on F1 films
| S. No. | Time in days | Physical changes | mean % drug release | ||
| Naratriptan | |||||
| 25 °C/60% | 30 °C/75% | 40 °C/75% | |||
| 1 | 01 | No Change | 94.62±0.12 | 94.62±0.22 | 94.62±0.081 |
| 2 | 30 | No Change | 93.10±0.112 | 93.24±0.24 | 93.24±0.19 |
| 3 | 60 | No Change | 92.58±0.273 | 92.49±0.19 | 92.52±0.22 |
| 4 | 90 | No Change | 91.99±0.04 | 91.52±0.02 | 91.20±0.05 |
Data is expressed as mean±SD; n = 3, (As the text states that exceeding 37 °C shows stability and yes it has shown instability by decreasing its release to 91.20% when compared to 25 °C, which shows release of 91.99%.)
CONCLUSION
From this study it was concluded that the buccal films containing naratriptan can be successfully prepared by using release rate controlling polymers. In the present study it can be concluded that FTIR studies revealed that there is no incompatibility or interaction between naratriptan and excipients. Formulated buccal films gives satisfactory film characteristics like physical appearance, surface texture, weight uniformity, thickness uniformity, folding endurance, surface pH, percentage swelling index, percentage moisture uptake, drug content uniformity, in vitro drug release. The low values for standard deviation for average weight, thickness, surface pH, percentage swelling index, percentage moisture uptake, in vitro drug release and drug content indicated uniformity within the batches Short-term stability studies of optimized formulation as per ICH guidelines indicated that there is no significant changes. Sofinally, it can be concluded that buccal films of naratriptan could provide sustained buccal delivery for prolonged period. A further clinical investigation has to be conducted to establish the safety and efficacy of the developed formulation.
ACKNOWLEDGEMENT
The authors would like to acknowledge each other’s support hard work and cooperation.
ABBREVIATIONS
DMSO-dimethylsulphoxide, Na CMC-sodium carboxymethyl cellulose, FTIR-fourier transform infrared spectroscopy, PEG-polyethylene glycol, RH-relative humidity
FUNDING
Nil
AUTHORS CONTRIBUTIONS
AA-Conceptualization, supervision and reviewing, MandFF-Writing, editing, reviewing.
CONFLICT OF INTERESTS
The authors declare no conflicts of interests.
REFERENCES
Salehi S, Boddohi S. New formulation and approach for mucoadhesive buccal film of rizatriptan benzoate. Prog Biomater. 2017;6(4):175-87. doi: 10.1007/s40204-017-0077-7, PMID 29110144.
Shin SC, Bum JP, Choi JS. Enhanced bioavailability by buccal administration of triamcinolone acetonide from the bio-adhesive gels in rabbits. Int J Pharm. 2009;209:37-43. doi: 10.1016/S0378-5173(00)00542-1.
Giradkar MA, Channawar AD, Kajale E, Sridhar RS, Kamble BV, Chandewar. Design development and in vitro evaluation of bioadhesive dosage form for buccal route. Int J Pharm Res Dev. 2010;2(6):1-20.
Mashru R, Sutariya V, Sankalia M, Sankalia J. Transbuccal delivery of lamotrigine across procine buccal mucosa in vitro determination of routes of buccal transport. J Pharm Pharm Sci. 2005 Feb 28;8(1):54-62. PMID 15946598.
Salamat Miller N, Chittchang M, Johnston TP. The use of mucoadhesive polymers in buccal drug delivery. Adv Drug Deliv Rev. 2005;57(11):1666-91. doi: 10.1016/j.addr.2005.07.003, PMID 16183164.
Begum MY, Alqahtani A, Ghazwani M, Ramakrishna MM, Hani U, Atiya A. Preparation of Carbopol 934-based ketorolac tromethamine buccal mucoadhesive film: in vitro, ex vivo, and in vivo assessments. Int J Polym Sci. 2021;2021:1-11. doi: 10.1155/2021/4786488.
Pramodkumar TM. Oral transmucosal drug delivery systems. Indian Drugs. 2004;41(2):63-1.
Sudhakar Y, Kuotsu K, Bandyopadhyay AK. Buccal bioadhesive drug delivery a promising option for orally less efficient drugs. J Control Release. 2006;114(1):15-40. doi: 10.1016/j.jconrel.2006.04.012, PMID 16828915.
Sudhakar Y, Kuotsu K, Bandyopadhyay AK. Buccal bioadhesive drug delivery a promising option for orally less efficient drugs. J Control Rel. 2006;114(1):15-40. doi: 10.1016/j.jconrel.2006.04.012.
Dixit GR. Formulation and characterization of mucoadhesive buccal film of ranitidine hydrochloride using Sterculia foetida gum as polymer. Asian J Pharm Clin Res. 2015;8(3):68-71.
Yelave A, Geetabhagwat. Mucoadhesive buccal films: a novel approach for the delivery of anti-hypertensive drugs. Asian J Pharm Clin Res. 2021;14(4):12-21. doi: 10.22159/ajpcr.2021.v14i4.40654.
Bobade NN. A review on buccal drug delivery system. Int J Pharm Pharm Sci Res. 2013;3(1):35-40.
Miller NS, Chittchang M, Johnston TP. The use of mucoadhesive polymers in buccal drug delivery. Adv Drug Deli Rev. 2005;4(2):1666-91.
Dixit RP, Puthli SP. Oral strip technology: overview and future potential. J Control Release. 2009;139(2):94-107. doi: 10.1016/j.jconrel.2009.06.014, PMID 19559740.
Khobragade PK, Puranik PK, Suradkhar SS. Literature studies on preparation and evaluation of buccal patches. Int J Pharm Rev Res. 2014;25(2):83-91.
Sindhu GD. Buccal mucoadhesive films. Int J Curr Pharm Res. 2021;13(4):14-20. doi: 10.22159/ijcpr.2021v13i4.42735.
Gawas S, Dev A, Deshmukh G, Rathod S. Current approaches in buccal drug delivery system. Pharm Biol Eval. 2016;3(2):165-7.
Patel R, Shardul N, Patel J, Baria. An overview on buccal mucoadhesive films. Arch PharmSci Res. 2009;1(2):212-7.
Amir H. Systemic drug delivery via the buccal mucosal route. Pharm Technol. 2001:1-27.
Apoorva M, Neha C, Geeta A. Formulation and characterization of fast dissolving buccal films: a review. Pharm Lett. 2011;3(1):152-65.
Gandhi RB, Robinson JR. Oral cavity as a site for bioadhesive drug delivery. Adv Drug Deliv Rev. 1994;13(1-2):43-74. doi: 10.1016/0169-409X(94)90026-4.
Prakash A, Soni PK, Paswan SK, Saini TR. Formulation and optimization of mucoadhesive buccal film for nicotine replacement therapy. Int J App Pharm. 2023;15(3):100-12. doi: 10.22159/ijap.2023v15i3.47412.
Nair AB, Shah J, Jacob S, Al Dhubiab BE, Patel V, Sreeharsha N. Development of mucoadhesive buccal film for rizatriptan: in vitro and in vivo evaluation. Pharmaceutics. 2021;13(5):728. doi: 10.3390/pharmaceutics13050728, PMID 34063402.
Muthamil C, Sreedharan NK, Senthilkumar KL. Formulation and evaluation of mucoadhesive film for the treatment of migraine. Int J Pharm Sci. 2024;2(5):660-72.
Nair AB, Al Dhubiab BE, Shah J, Jacob S, Saraiya V, Attimarad M. Mucoadhesive buccal film of almotriptan improved therapeutic delivery in rabbit model. Saudi Pharm J. 2020;28(2):201-9. doi: 10.1016/j.jsps.2019.11.022, PMID 32042259.
Bhikshapathi V, madhuri D, Rajesham V, R. Preparation and evaluation of fast dissolving oral film containing naratriptan HCl. Am J Pharm Tech Res. 2014;4(2):799-812.