
Int J Pharm Pharm Sci, Vol 17, Issue 10, 45-47Case Study
HIDDEN DANGER IN PSYCHIATRY: DRUG-INDUCED CHOLESTASIS AS AN UNDERRECOGNISED ADVERSE EFFECT
RENGARAJ THIRUNANAMOORTHY1, VENNILA SANKAR2, THASLIM RIDHWANA BARAKATH ALI2*, PARI KUMANAN2, SURYA RAJENDRAN2
1Department of General Medicine, Government Medical College and Hospital, Nagapattinam, Tamil Nadu, India. 2Department of Pharmacy Practice, E. G. S. Pillay College of Pharmacy, Nagapattinam, Tamil Nadu, India
*Corresponding author: Thaslim Ridhwana Barakath Ali; *Email: thaslimbarakath@gmail.com
Received: 16 Jul 2025, Revised and Accepted: 07 Aug 2025
ABSTRACT
Drug-induced liver injury (DILI) often goes overlooked due to its non-specific symptoms. If left untreated, even acute liver injury could lead to serious complications. We reported the case of a 50 y old female who presented with symptoms of acute gastroenteritis and urinary tract infection. Initially, liver function tests revealed elevated aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, and bilirubin levels. The patient’s history of multiple psychiatric medications: tablet chlorpromazine, tablet olanzapine, capsule fluoxetine, and tablet diazepam, raised suspicion of DILI. The updated Roussel Uclaf Causality Assessment Method (RUCAM) scoring indicated “possible” causality for all four drugs, with chlorpromazine receiving the highest score. All psychiatric medications were discontinued, and the repeated liver function test after eight days demonstrated significant improvement. This case highlighted the importance of liver function monitoring in patients receiving psychotropic polypharmacy and underscored the value of structured causality tools such as RUCAM in guiding clinical decisions.
Keywords: Drug-induced liver injury, Cholestatic liver injury, Psychiatric drugs, RUCAM, Adverse drug reactions, Pharmacovigilance
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijpps.2025v17i10.56102 Journal homepage: https://innovareacademics.in/journals/index.php/ijpps
INTRODUCTION
The liver is a vital organ, actively contributing to the detoxification of various drugs and xenobiotics. Despite its remarkable regenerative ability, it can be affected by the reactive drug metabolites and other toxic intermediates [1]. Liver injury can be caused due to various prescription, non-prescription or traditional drugs, and it is referred to as DILI. Globally, drug-induced liver injury (DILI) occurs in approximately one in 10,000 to one in 1,00,000 individuals annually, with sedatives and neuropsychiatric agents implicated in approximately 2.6% of cases [2]. However, accurate data on DILI incidence among the Indian population remains sparse [3].
The broadly accepted mechanisms behind DILI are direct liver toxicity and idiosyncratic responses to medication [2]. DILI can be presented in three well-defined forms – hepatocellular, cholestatic and mixed. Cholestatic (impaired bile flow) liver injury marks 20-40% of DILI by a significant increase in alkaline phosphatase (ALP) and a slight elevation in alanine aminotransferase (ALT), and occasional increase in bilirubin levels [4].
Psychiatric medications of various categories, like antidepressants (e. g., fluoxetine), antipsychotics (e. g., olanzapine, chlorpromazine) and benzodiazepines (e. g., diazepam) have been associated and reported to cause DILI. Mostly, DILI is reversible on withdrawal, but rare cases of liver failure, transplantation or mortality are also documented [5].
This report is a case of asymptomatic cholestastic liver injury developed after initiation of four psychiatric drugs: Chlorpromazine, olanzapine, fluoxetine and diazepam, highlighting the need for regular monitoring of DILI in patients undergoing psychiatric polypharmacy.
Case presentation
A fifty-year-old female patient is admitted to the female medicine ward with the chief complaints of vomiting (ten episodes) and diarrhea (six episodes) for two days. The patient’s complaint extended to mild fever and burning micturition. She was presented with angular cheilitis during admission. On examining the vitals of the patient during admission, the blood pressure was noted to be 140/90 mmHg, and the pulse rate was 72 beats per minute. The complete blood count showed results of elevated white blood cells (14.4 X 109/l) and platelets (448 X 109/l) consistent with infection. She was diagnosed with acute gastroenteritis and a urinary tract infection.
The abnormal results of liver function tests (LFT), despite the patient being asymptomatic for hepatic illness, provided a different angle to this case. On day 1, before initiating the treatment for acute gastroenteritis and urinary tract infection, the results of LFT showed elevated aspartate aminotransferase (AST), ALT, ALP and bilirubin levels. She had no history of alcohol consumption or concomitant liver diseases. There were no signs of clinical jaundice, right upper quadrant tenderness, hepatomegaly and viral markers (HBsAg and anti-HCV) were negative.
Her past medical history revealed severe depression since 2021. She has been on a course of psychiatric medications, which involved Tab. Olanzapine 5 mg at bedtime, two tablets of diazepam 5 mg at bedtime and Capsule fluoxetine 20 mg in the morning for the last six months. From the last three months, Tab. Chlorpromazine 25 mg at bedtime has been added to her psychiatric medications due to her complaints of sleep disturbances, palpitations and loss of appetite.
Table 1: Comparing liver function test values on Day 1 and Day 8
| Parameter | Day 1 | Day 8 |
| AST | 189 U/l | 86 U/l |
| ALT | 50 U/l | 33 U/l |
| ALP | 192 U/l | 78 U/l |
| Total Bilirubin | 1.58 mg/dl | 0.79 mg/dl |
| Direct Bilirubin | 0.50 mg/dl | 0.27 mg/dl |
| Indirect Bilirubin | 1.08 mg/dl | 0.52 mg/dl |
The first day's findings led to the suspicion of drug-induced cholestatic liver injury. Immediate cessation of all four psychiatric drugs was done, and the patient was started on Ursodeoxycholic acid 300 mg once daily in tablet form. Treatment for acute gastroenteritis and urinary tract infection was also given with Inj. Ciprofloxacin 200 mg (IV, twice daily), Inj. Metronidazole 500 mg (IV, three times a day), Inj. Ranitidine 50 mg (IV, twice daily), Inj. Ondansetron 4 mg once daily, capsule doxycycline 100 mg twice daily, tablet zinc 10 mg twice daily, probiotic capsules and oral rehydration solution.
The patient remained stable and was discharged in her recovery phase (Day 8). No further documentation on follow-up was available.
DISCUSSION
DILI is a serious negative drug reaction that needs attention, particularly in psychiatric patients exposed to polypharmacy for a long time [3]. Previous data suggest idiosyncratic DILI to be a rare condition. However, DILI is one of the under-reported and overlooked clinical syndromes due to the lack of a proper surveillance mechanism in monitoring DILI, leading to the debate of its incidence [6]. The lack of accurate DILI data in Indian settings and the ignorance of previously documented DILI highlight the need for creating awareness on evaluation and risk factors causing DILI.
The RUCAM scoring system is a standardised and validated tool for assessing the causality relationship between DILI and the suspected drugs [7]. Previous studies have also emphasized close monitoring of liver function in patients exposed to psychotropic drugs like antidepressants [8].
The prerequisite to undergo RUCAM is to classify the DILI into the hepatocellular, cholestatic or mixed type of injury. This classification can be achieved by calculating the R ratio using the following formula [4],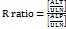
Assuming the upper limits of normal [ULN] are 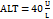 and
and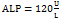 ,
,
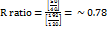 .
.
The calculated R value indicated a cholestatic pattern of liver injury [7].
Table 2: Causality assessment of chlorpromazine for DILI using RUCAM
| Characteristics | Score | Justification |
| Time of onset | +2 | The reaction was found within 90 days of treatment with Tab. Chlorpromazine 25 mg at bedtime |
| After cessation of the drug | +2 | Decrease in ALP levels more than 50% on the eighth day after stopping the suspected drug |
| Risk factors | 0 | No history of alcohol use and the age of the patient is below 55 |
| Concomitant drugs | -1 | Concomitant drugs like Tab. Fluoxetine, Tab. Olanzapine and Tab. Diazepam was taken from 6 mo |
| Exclusion of other causes | 0 | Hepatitis B virus, Hepatitis C virus, alcoholism and recent history of concomitant diseases were ruled out |
| Previous information on hepatotoxicity of the drug | +2 | Reaction labelled in the product characteristics |
| Re-administration | 0 | Not done |
| Total RUCAM Score | +5 | Possible |
Table 3: Causality assessment of olanzapine, fluoxetine and diazepam for DILI using RUCAM
| Characteristics | Score | Justification |
| Time of onset | +1 | The reaction was found after 90 d of treatment with Tab. Olanzapine, Cap. Fluoxetine 20 mgin morning and Tab. Diazepam 10 mg at bedtime |
| After cessation of the drug | +2 | Decrease in ALP levels more than 50% on the eight day after stopping the suspected drug |
| Risk factors | 0 | No history of alcohol use and the age of the patient is below 55 |
| Concomitant drugs | -1 | Concomitant drug, Tab. Chlorpromazine was taken from three months. |
| Exclusion of other causes | 0 | Hepatitis B virus, Hepatitis C virus, alcoholism and recent history of concomitant diseases were ruled out |
| Previous information on hepatotoxicity of the drug | +2 | Reaction labelled in the product characteristics |
| Re-administration | 0 | Not done |
| Total RUCAM Score | +4 | Possible |
According to the RUCAM standard scoring method, all four psychiatric drugs, i. e., chlorpromazine, olanzapine, fluoxetine, and diazepam, fall under the ‘possible’ causality category. Among them, tablet chlorpromazine obtained the highest score, reflecting a higher causality relationship, suggesting it is the most likely offending agent.
There is no standard protocol for treating DILI. Instead, higher importance is given to the withdrawal of suspected drugs. In chronic psychiatric conditions, cessation of all the drugs becomes difficult. In this case, all four drugs were temporarily stopped and the physician recommended cessation of only Tab. Chlorpromazine 25 mg at bedtime after consultation with the psychiatric doctor. The patient was advised to have a follow-up visit after a month for evaluating subsequent LFT assessment, re-evaluation of psychiatric medications would be considered if necessary [4].
Treatment with Ursodeoxycholic acid tablet (300 mg, twice a day) was also started. This drug offers hepatoprotective effects, primarily by reducing bile acid-induced cytotoxicity [4].
Treatment with Inj. Ciprofloxacin 200 mg (IV, twice daily), Inj. Metronidazole 500 mg (IV, three times a day), Inj. Ranitidine 50 mg (IV, twice daily), Inj. Ondansetron 4 mg once daily, capsule doxycycline 100 mg twice daily, tablet zinc 10 mg twice daily, probiotic capsules and oral rehydration solution for managing acute gastroenteritis and urinary tract infection were administered to the patient only after obtaining her baseline LFT, which had already shown abnormalities, thereby ruling out the possibility of these drugs causing DILI.
Although none of the four psychotropic agents achieved a probable or highly probable causality, the psychotropic poly-pharmacy with all the drugs known to have hepatotoxic potential created a diagnostic challenge that underscores the importance of regular liver function monitoring.
CONCLUSION
This case highlights the importance of vigilance when prescribing multiple psychotropic agents, particularly when all the prescribed drug has the potential to cause hepatotoxicity. It highlights the importance of routine liver function monitoring even when the patient shows no symptoms of liver impairment. The necessity of tools like RUCAM in guiding clinical decisions is also highlighted here.
Although the limitations of this study, like lack of rechallenge, follow up or imaging, could not find the exact culprit, this case implies the need for early recognition and medically supervised withdrawal of the suspected drugs to prevent liver damage and facilitate recovery.
ACKNOWLEDGEMENT
The authors gratefully acknowledge and express their sincere gratitude for the support provided by Government Medical College and Hospital, Nagapattinam, and the efforts of the treating team involved in the patient’s care.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
Rengaraj Thirunanamoorthy supervised the clinical execution at the hospital, ensured ethical compliance and provided final approval for the manuscript. Vennila Sankar contributed to the literature search, preparation of the informed consent form, data collection, and offered critical inputs on the manuscript. Thaslim Ridhwana Barakath Ali was involved in conceptualization, literature search, data collection, preparing of the initial draft, and manuscript revision. Pari Kumanan assisted with data collection, patient follow-up, and reviewed the draft. Surya Rajendran contributed to the literature review, manuscript editing, and formatting.
CONFLICT OF INTERESTS
Declared none
REFERENCES
Dey P, Saha MR, Sen A. An overview on drug-induced hepatotoxicity. Asian J Pharm Clin Res. 2013;6(4):1-4.
LV Q, Yi Z. Antipsychotic drugs and liver injury. Shanghai Arch Psychiatry. 2018;30(1):47-51. doi: 10.11919/j.issn.1002-0829.217090, PMID 29719358.
Subramaniam B, Shah M, Desai C, Panchal J, Shah S. An analysis of cases of drug-induced liver injury reported to an adverse drug reaction monitoring center. Asian J Pharm Clin Res. 2020;13(11):109-12. doi: 10.22159/ajpcr.2020.v13i11.39312.
Sundaram V, Bjornsson ES. Drug-induced cholestasis. Hepatol Commun. 2017;1(8):726-35. doi: 10.1002/hep4.1088, PMID 29404489.
Telles Correia D, Barbosa A, Cortez Pinto H, Campos C, Rocha NB, Machado S. Psychotropic drugs and liver disease: a critical review of pharmacokinetics and liver toxicity. World J Gastrointest Pharmacol Ther. 2017;8(1):26-38. doi: 10.4292/wjgpt.v8.i1.26, PMID 28217372.
Chalasani N, Bjornsson E. Risk factors for idiosyncratic drug-induced liver injury. Gastroenterology. 2010;138(7):2246-59. doi: 10.1053/j.gastro.2010.04.001, PMID 20394749.
Danan G, Teschke R. Rucam in drug and herb-induced liver injury: the update. Int J Mol Sci. 2015;17(1):14. doi: 10.3390/ijms17010014, PMID 26712744.
Lu LY, Tsai CC. Analysis of agomelatine treatment with depressive symptoms. Int J App Pharm. 2021;13Suppl 1:63-6. doi: 10.22159/ijap.2021.v13s1.Y0110.